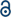 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Spray-coating polystyrene on perovskite solar cells increases thermal stability and moisture tolerance†
Azar
Sadollahkhani‡
,
Valentina
Leandri‡
,
Mahboubeh
Jamshidi
 and
James M.
Gardner
and
James M.
Gardner
 *
*
Division of Applied Physical Chemistry, Department of Chemistry, KTH Royal Institute of Technology, SE−100 44, Stockholm, Sweden. E-mail: [email protected]; Tel: +46 8 790 81 67
First published on 20th March 2025
Abstract
The primary challenge for the commercialization of hybrid perovskite solar cells (PSCs) is their chemical and thermal instability as compared to Si devices. Herein, we demonstrate that PSCs spray-coated with polystyrene retain 80% of their efficiency after 40 hours immersed in water or at 95 °C.
Sustainability spotlightPerovskite solar cells (PSCs) are energy-efficient alternatives to traditional silicon PVs. However, PSCs have their own environmental challenges: susceptibility to moisture, heat, and UV, and concerns about Pb(II) leakage. One promising solution is polymer coatings, like polystyrene, which act as a protective barrier. Coatings not only reduce Pb(II) diffusion but also enhance cell durability. Polystyrene repels water but reacts easily with acetone, allowing selective access to Pb(II) for recycling at the end of the solar cell's life cycle, further supporting sustainable practices in renewable energy. Our work emphasizes the UN Sustainable Development Goal (SDG) 12 on responsible consumption and production, which includes the specific target on responsible management of chemicals. The work also contributes to: affordable and clean energy (SDG 7), industry, innovation, infrastructure (SDG 9), and climate action (SDG 13). |
Hybrid organic–inorganic halide perovskite solar cells (PSCs) have shown rapid progress in power conversion efficiency (PCE), from initial reports of 2–3% in 2009 to a certified efficiency of 26.7% in 2024.1–5 They represent a potential earth-abundant and low-energy-production alternative to silicon solar cells.6,7 Although PSCs have achieved high efficiencies in a very short time, one of the major barriers to their commercialization is the poor material and device stability.8–10
Among the various perovskite absorber layers, methylammonium lead iodide (MAPbI3) is the most extensively researched hybrid organic–inorganic perovskite for PSC applications. MAPbI3 offers several unique properties and advantages, making it suitable for use in PSCs. It has an optimal bandgap of approximately 1.6 eV and a high absorption coefficient (in the range of 104 to 105 cm−1), which is more than an order of magnitude greater than that of silicon in the visible light spectrum.11 However, MAPbI3 faces significant issues with stability, posing a major obstacle to the commercialization of MAPbI3-based perovskite solar cells (PSCs).12 In this regard, mixed-cation, mixed-halide perovskites (e.g., [(CH(NH2)2PbI3)0.85(CH3NH3PbBr3)0.15]) and 3D–2D perovskites with long alkyl chains have enhanced moisture tolerance and stability compared to the pure CH3NH3PbI3 perovskite.3,13–18 Additionally, in order to improve the stability of PSCs, several studies have proposed the use of hydrophobic polymers as water and moisture barriers with promising results in different solar cells.19,20 Some examples are as follows: Chen et al. used polystyrene microgel particles to increase PSC stability and replace the majority of the HTM phase.21 Bella et al. applied a fluorinated polymer that contains pigments to absorb ultraviolet light and re-emit it in the visible range which can boost cell efficiency and limit photodegradation.22 Bi et al. incorporated PMMA into the perovskite film as a template to control nucleation and crystallization processes, achieving a certified PCE of 21.02%.23 Meng and colleagues employed PTQ10 to minimize the loss of surface organic cations and regulate the kinetics of phase conversion.24 Zhao et al. proposed an innovative polymerization-assisted grain growth strategy, which effectively passivated undercoordinated lead ions while improving operational stability.25 Recently, Wang et al. incorporated the hydrophobic polymer coenzyme Q10 into perovskite films which enabled 22.73% efficiency and 91% retention after 1000 hours of ambient storage without encapsulation.26 Moreover, Raman and coworkers investigated scalable bilayer polymer encapsulation. They employed bilayer poly(methyl methacrylate)/polyurethane (PMMA/PU) and fabricated devices which retained more than 92% of their initial performance after 1500 h (60 days).27
The present work focuses on the application and use of polystyrene in PSCs. Sonu et al. treated carbon electrodes in perovskite solar cells with a toluene solution containing polystyrene. The polystyrene improved charge transfer, reduced recombination, and enhanced stability against light and moisture. This treatment boosted the power conversion efficiency (PCE) from 11.31% to 14.03%.28 Another investigation focused on a triphenylamine (TPA) and polystyrene blend used as an interfacial layer in PSCs. This blend effectively suppressed energy losses and improved device stability by passivating surface defects in the perovskite layer.29
These studies underscore the significant role of polymers in enhancing the performance of PSCs. Following a similar approach, we present regular n–i–p PSCs (glass/FTO/TiO2/perovskite/Spiro-OMeTAD/Au) with remarkably improved stability after spray-coating a layer of polystyrene. The choice of the relatively low molecular weight polymer used is tied to the necessity of using a solvent which does not cause damage to any of the layers present in the solar cells when sprayed on top of the devices/films. We found that cyclohexane does not cause damage to any of the layers due to the lack of solubility of the components in the solvent. On the other hand, this polymer can be dissolved in acetone and recycles when the lifetime of the solar cell is over.
In order to increase the knowledge about the stability of pure CH3NH3PbI3 solar cells, both mixed-cation, mixed-halide [(CH(NH2)2PbI3)0.85(CH3NH3PbBr3)0.15] and pure (CH3NH3PbI3) perovskites were employed for the fabrication of photovoltaic devices and their stability was investigated under different conditions. To the best of our knowledge, the results presented in this paper show one of the highest water tolerances reported for perovskite solar cells. We show that the polystyrene coating protects the active layers of the devices from water permeation by demonstrating coated devices that retain ∼90% of their initial power conversion efficiency after 20 hours of immersion in water, and ∼50% after 110 hours. Moreover, the perovskite films coated with the polystyrene layer display a remarkable thermal stability, suggesting a good intrinsic stability of the materials. Furthermore, by monitoring the performance of the devices under light exposure, we show that the polystyrene coating decreases the light-assisted degradation rate of perovskite solar cells that use CH3NH3PbI3 as a light-harvesting layer leading to a sustainable solution.
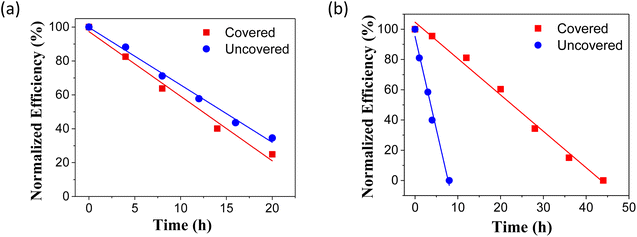
In the initial investigation the hydrophobic film was developed onto the complete device using the spraying method with an airbrush (Fig. S1 and S2†). Four different groups of devices were prepared and immersed in deionized water: mixed perovskite covered/uncovered, and pure perovskites covered/uncovered. The efficiency of the devices was monitored over time and the results are collected in Fig. 1.
The impact of the polystyrene coating on the photovoltaic performance of PSCs has been evaluated, and the results are reported in the ESI (Fig. S3 and S4).†
Uncovered solar cells started to degrade immediately after their immersion in water and fully decomposed within 10 minutes. The solar cells protected by the polystyrene coating showed remarkable stability. After 20 h immersion in water, both the covered mixed and pure devices retained ∼90% of their initial efficiency, and after 110 h immersion in water the response is still ∼50%. Among laboratory solar cells with a similar structure, this performance demonstrates a notable level of water tolerance. The loss of performance over immersion time followed the same trend for the covered mixed and pure devices under investigation, suggesting its sole dependence from water intrusion. The rapid colour change of the uncovered devices is attributed to the decomposition of the perovskite crystal structure into the starting materials.10 This structural change is initiated by the introduction of water molecules into the perovskite layer, where they form weak hydrogen bonds to the highly hygroscopic methylammonium cations, causing a bond dissociation between the crystal constituents. As a consequence, the unbound methylammonium iodide can readily escape the crystal structure, leading to a residual layer of PbI2. This is a rapid process for uncovered devices while, for devices covered with a hydrophobic polymer layer, the water penetration can be substantially delayed, slowing down the degradation mechanism. Fig. S5 in the ESI† illustrates the time-dependent changes in photovoltaic parameters during water immersion.
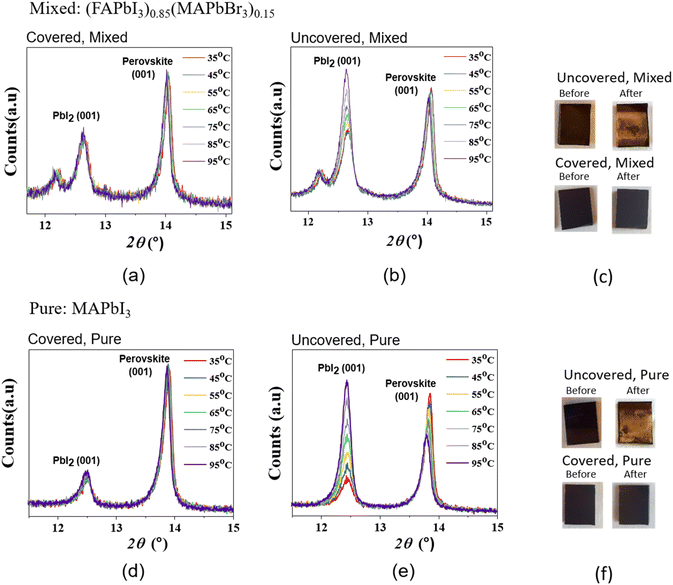
In order to investigate the effect of the polystyrene coating on the thermal and moisture stability of the perovskite films, the substrates were subjected to thermal stress through a wide range of temperatures, from 35 to 95 °C in an ambient atmosphere with relative humidity >40%, and their X-ray diffraction patterns have been recorded. The results are summarized in Fig. 2. Fig. 2a and d clearly show the stability of the covered perovskite substrates during the experiment. Meanwhile, Fig. 2b and e display the decomposition of the materials, which is expressed as an enhancement of the diffraction peak related to the free PbI2, and a decrease of the signal related to the (001) crystal plane of the perovskite structure.
A neat colour change, from dark brown to yellow, was also observed for both the mixed and the pure uncovered perovskite samples (Fig. 2c and f) at the end of the experiments, while no colour change was observed for the corresponding covered samples. As previously mentioned, the colour change is associated with the decomposition of the perovskite structure and these observations are consistent with the X-ray analysis of the substrates.
To quantitatively compare the transformation from perovskite to PbI2, the ratios of the maximum intensities for the PbI2 (001)/perovskite (001) peaks are shown in Table 1.
| Perovskite | 35 °C | 45 °C | 55 °C | 65 °C | 75 °C | 85 °C | 95 °C |
|---|---|---|---|---|---|---|---|
| a M-U: mixed perovskite film, uncovered. b M-C: mixed perovskite film, covered. c P-U: pure perovskite film, uncovered. d P-C: pure perovskite film, covered. | |||||||
| M-Ua | 0.59 | 0.63 | 0.64 | 0.66 | 0.75 | 0.86 | 1.07 |
| M-Cb | 0.53 | 0.53 | 0.53 | 0.54 | 0.54 | 0.55 | 0.57 |
| P-Uc | 0.41 | 0.53 | 0.66 | 0.87 | 1.02 | 1.30 | 1.55 |
| P-Cd | 0.41 | 0.41 | 0.41 | 0.41 | 0.41 | 0.42 | 0.44 |
The results show that for the uncovered mixed perovskite the ratio of the intensity for PbI2 (001)/perovskite (001) doubles as temperature increases from 35 to 95 °C, while for the uncovered pure perovskite this number increases to four times the original PbI2 (001)/perovskite (001) ratio. Nevertheless, for the covered samples (both pure and mixed) the ratio remains nearly constant at all tested temperatures. We believe that the decomposition process occurring in the uncovered substrates could be ascribed to the loss of the organic cations to the gas phase as neutral molecules and HI/HBr acids. Consequently, the covered samples do not degrade as the polar gases formed have no place to go when they encounter the nonpolar polymer layer. Additionally, as the formation of the decomposition gases is catalysed by water, it is reasonable to assume that a hydrophobic barrier, such as the polystyrene layer, can reduce moisture penetration into the perovskite layer thus improving stability.
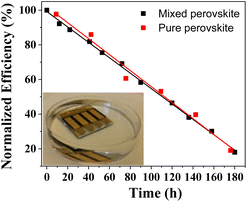
In the interest of studying the effect of polystyrene coating on the light-assisted degradation of mixed and pure perovskite solar cells, complete devices were continuously irradiated by white light with simulated 1 Sun intensity under open circuit conditions. The photovoltaic details were periodically measured, and the results are shown in Fig. 3. Fig. 3a shows the results for covered and uncovered mixed perovskite solar cells and demonstrates a significant degradation in the performance for all devices regardless of coating treatment. In contrast to the mixed perovskite solar cells, covered pure perovskite solar cells (Fig. 3b) show a significant improvement in stability when compared to the uncovered cells. The degradation of the perovskite solar cells upon exposure to light can occur for a variety of reasons. Photo-induced oxidation of Spiro, which can be accelerated through doping with LiTFSI and TBP can be considered as one of the main degradation mechanisms occurring in perovskite solar cells.30 It is reported that, from the power-to-current efficiency point of view, there is an oxidation threshold for Spiro-OMeTAD and that, when crossing that point, the performance of the device starts dropping dramatically.31 It is obvious that the polymer coating cannot have any effect on photo-induced oxidation of Spiro-OMeTAD and, consequently, it does not make any improvement regarding the degradation of a hole-transporting material. Nonetheless, the results show that, for pure perovskite solar cells, the polymer coating results in a significant improvement towards light stability. We suggest that, besides the photo-induced degradation of the hole-transporting material and the degradation induced by moisture, which occur equally in both pure and mixed perovskites, these materials suffer from different photochemical degradation mechanisms.32 Photo-induced phase segregation can cause iodide and bromide anions to migrate within the perovskite crystals and results in dramatic losses in solar cell performance.33 The phase segregation is anticipated to occur in both the covered and uncovered mixed perovskites, which is consistent with our observations.
The investigation of the different light-induced degradation mechanisms occurring in the pure and mixed perovskite is beyond the scope of this contribution. However, from the results presented in Fig. 3 and from an analysis of the detailed photovoltaic parameters under light exposure (Fig. S6†), one can conclude that in the case of the mixed perovskite solar cells, the photochemically induced degradation mechanisms are serious and more destructive than the moisture-assisted degradation.34,35 Fig. S5 and S6† demonstrate that the covered solar cells exposed to moisture and illumination suffer losses in efficiency primarily from decreases in photocurrent. This may be attributed to decomposition reactions originating at the perovskite interfaces.14 An increase in interfacial charge-transfer resistance due to the formation of PbI2, would negatively impact the photocurrent but not impact open-circuit voltage.
In conclusion, a polystyrene layer was spray coated as a protective hydrophobic barrier onto the hole-transporting material side of regular planar (n-i-p) PSCs. Due to the hydrophobicity of polystyrene, the coated solar cells repelled water from both the ambient atmosphere and direct contact, resulting in a significantly enhanced stability towards decomposition. The perovskite solar cells without the polystyrene layer are fully decomposed within 10 min and the colour of the devices changed from dark brown to yellow, indicating the formation of a PbI2 film. In contrast, the polystyrene-treated perovskite solar cells lost only 10% of their original efficiency after 20 h of immersion in water. The temperature and moisture-assisted degradation were investigated by measuring the XRD patterns of covered and uncovered perovskite films. The perovskite films without polystyrene exhibited a marked decomposition into PbI2 upon increasing the temperature from 35 °C to 95 °C in a relative humidity >40%, while the covered devices displayed no noticeable change in the XRD patterns. The light and moisture-assisted decomposition of mixed and pure perovskite solar cells showed different trends for the covered and uncovered devices. For the pure perovskite the results demonstrated that untreated solar cells decompose completely after 8 h continuous light exposure, whereas the covered devices retain 91% of the initial efficiency after 8 h. For the mixed perovskite devices, the difference between the device performance for covered and uncovered solar cells was negligible and within experimental error. The latter finding demonstrates that covering devices with polystyrene does not have any effect on the light-assisted degradation of mixed perovskite solar cells and suggests a photochemical degradation path which is not affected by moisture. Additionally, the spray coating method employed in this study is performed at room temperature, has high homogeneity, and is scalable from a laboratory framework to an industrial-scale of roll-to-roll processing. The spray coating of polystyrene onto perovskites demonstrates that inexpensive, scalable methods may be used to produce resilient perovskite solar cells. Finally, since polystyrene is soluble in acetone, it can be removed from the solar cell and recycled after the solar cell's lifetime.
Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was funded by “STandUP for ENERGY”, the Swedish Energy Agency, the Ragnar Holm Foundation, and SSF Swedish Foundation for Strategic Research.Notes and references
- National Renewable Energy Laboratory in Golden, Colorado, https://www.nrel.gov/pv/cell-efficiency.html CAS.
- Z. Guo, et al., Long-range hot-carrier transport in hybrid perovskites visualized by ultrafast microscopy, Science, 2017, 356(6333), 59–62 CrossRef CAS PubMed.
- Y. Sun, et al., Triple-cation mixed-halide perovskites: towards efficient, annealing-free and air-stable solar cells enabled by Pb(SCN)2 additive, Sci. Rep., 2017, 7(1), 46193 CrossRef CAS.
- W. S. Yang, et al., Iodide management in formamidinium-lead-halide–based perovskite layers for efficient solar cells, Science, 2017, 356(6345), 1376–1379 CAS.
- S. Liu, et al., Buried interface molecular hybrid for inverted perovskite solar cells, Nature, 2024, 632(8025), 536–542 Search PubMed.
- S. N. Habisreutinger, et al., Research Update: Strategies for improving the stability of perovskite solar cells, APL Mater., 2016, 4(9), 091503 Search PubMed.
- A. Machín and F. Márquez, Advancements in Photovoltaic Cell Materials: silicon, organic, and Perovskite Solar Cells, Materials, 2024, 17(5), 1165 Search PubMed.
- W. Xiang, S. Liu and W. Tress, A review on the stability of inorganic metal halide perovskites: challenges and opportunities for stable solar cells, Energy Environ. Sci., 2021, 14(4), 2090–2113 CAS.
- J. Yang and T. L. Kelly, Decomposition and Cell Failure Mechanisms in Lead Halide Perovskite Solar Cells, Inorg. Chem., 2017, 56(1), 92–101 CAS.
- J. Huang, et al., Impact of H2O on organic–inorganic hybrid perovskite solar cells, Energy Environ. Sci., 2017, 10(11), 2284–2311 RSC.
- T. T. Ava, et al., A Review: Thermal Stability of Methylammonium Lead Halide Based Perovskite Solar Cells, Appl. Sci., 2019, 9(1), 188 CAS.
- B. Dahal and W. Li, Configuration of Methylammonium Lead Iodide Perovskite Solar Cell and its Effect on the Device's Performance: A Review, Adv. Mater. Interfaces, 2022, 9(19), 2200042 CAS.
- B. P. Kore, et al., Moisture tolerant solar cells by encapsulating 3D perovskite with long-chain alkylammonium cation-based 2D perovskite, Commun. Mater., 2021, 2(1), 100 CAS.
- B. P. Kore, M. Jamshidi and J. M. Gardner, The impact of moisture on the stability and degradation of perovskites in solar cells, Mater. Adv., 2024, 5(6), 2200–2217 CAS.
- P. Wu, et al., Mixed Cations Enabled Combined Bulk and Interfacial Passivation for Efficient and Stable Perovskite Solar Cells, Nano–Micro Lett., 2023, 15(1), 114 CAS.
- J. Hieulle, et al., Metal Halide Perovskite Surfaces with Mixed A-Site Cations: Atomic Structure and Device Stability, Adv. Funct. Mater., 2023, 33(9), 2211097 CrossRef CAS.
- L. K. Ono, E. J. Juarez-Perez and Y. Qi, Progress on Perovskite Materials and Solar Cells with Mixed Cations and Halide Anions, ACS Appl. Mater. Interfaces, 2017, 9(36), 30197–30246 CAS.
- S. Dai, et al., Pure-phase two-dimensional perovskite capping layer enables high-performance and durable carbon-based photovoltaics, Chem. Eng. J., 2024, 497, 154611 CrossRef CAS.
- S. Wang, et al., Polymer strategies for high-efficiency and stable perovskite solar cells, Nano Energy, 2021, 82, 105712 CrossRef CAS.
- S. Wu, et al., Multifunctional Two-Dimensional Benzodifuran-Based Polymer for Eco-Friendly Perovskite Solar Cells Featuring High Stability, ACS Appl. Mater. Interfaces, 2022, 14(36), 41389–41399 CrossRef CAS.
- M. Chen, et al., Reducing hole transporter use and increasing perovskite solar cell stability with dual-role polystyrene microgel particles, Nanoscale, 2017, 9(28), 10126–10137 Search PubMed.
- F. Bella, et al., Improving efficiency and stability of perovskite solar cells with photocurable fluoropolymers, Science, 2016, 354(6309), 203–206 CAS.
- D. Bi, et al., Polymer-templated nucleation and crystal growth of perovskite films for solar cells with efficiency greater than 21%, Nat. Energy, 2016, 1(10), 16142 CAS.
- L. Meng, et al., Tailored Phase Conversion under Conjugated Polymer Enables Thermally Stable Perovskite Solar Cells with Efficiency Exceeding 21%, J. Am. Chem. Soc., 2018, 140(49), 17255–17262 CAS.
- Y. Zhao, et al., A Polymerization-Assisted Grain Growth Strategy for Efficient and Stable Perovskite Solar Cells, Adv. Mater., 2020, 32(17), 1907769 CAS.
- J. Wang, et al., Synergistic Tailoring Crystallization and Enhancing Stability by a Hydrophobic Polymer Enables High-Performance Perovskite Solar Cells, ACS Appl. Energy Mater., 2024, 7(9), 3841–3847 CAS.
- R. K. Raman, et al., Facile and scalable bilayer polymer encapsulation to achieve long-term stability of perovskite solar cells under harsh humidity conditions, Sustainable Energy Fuels, 2024, 8(9), 1953–1965 RSC.
- K. S. Sonu, et al., Effect of polystyrene treatment on the efficiency and stability of fully printable mesoscopic perovskite solar cells with carbon electrode, J. Mater. Sci.: Mater. Electron., 2021, 32(10), 13440–13449 CrossRef.
- J. Ran, et al., Triphenylamine–Polystyrene Blends for Perovskite Solar Cells with Simultaneous Energy Loss Suppression and Stability Improvement, Sol. RRL, 2020, 4(12), 2000490 CAS.
- R. S. Sanchez and E. Mas-Marza, Light-induced effects on Spiro-OMeTAD films and hybrid lead halide perovskite solar cells, Sol. Energy Mater. Sol. Cells, 2016, 158, 189–194 CAS.
- O. A. Jaramillo-Quintero, et al., Bright Visible-Infrared Light Emitting Diodes Based on Hybrid Halide Perovskite with Spiro-OMeTAD as a Hole-Injecting Layer, J. Phys. Chem. Lett., 2015, 6(10), 1883–1890 CAS.
- U. B. Cappel, et al., Partially Reversible Photoinduced Chemical Changes in a Mixed-Ion Perovskite Material for Solar Cells, ACS Appl. Mater. Interfaces, 2017, 9(40), 34970–34978 CAS.
- G. F. Samu, C. Janáky and P. V. Kamat, A Victim of Halide Ion Segregation. How Light Soaking Affects Solar Cell Performance of Mixed Halide Lead Perovskites, ACS Energy Lett., 2017, 2(8), 1860–1861 CAS.
- R. K. Misra, et al., Effect of Halide Composition on the Photochemical Stability of Perovskite Photovoltaic Materials, ChemSusChem, 2016, 9(18), 2572–2577 CAS.
- A. Sadollahkhani, et al., Energetic Barriers to Interfacial Charge Transfer and Ion Movement in Perovskite Solar Cells, ChemPhysChem, 2017, 18(21), 3047–3055 CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: The following are available online: Fig. S1: schematic representation of the spray coating method used in this manuscript, Fig. S2: J–V responses under simulated 1 sun illumination for mixed and pure perovskite solar cells, Table S1: champion device performances under simulated AM 1.5 G solar illumination for the mixed and pure perovskite solar cells reported in Fig. S1 and S3: J–V responses under simulated 1 sun illumination for the (a) mixed perovskite solar cell and (b) pure perovskite solar cell before (black line) and after (red line) polymer coating, Table S2: device performance under simulated AM 1.5 G solar illumination of the mixed and pure perovskite solar cells in Fig. S2, before and after polymer coating, Fig. S4: efficiency of 16 different devices based on mixed (a) and pure (b) perovskites, before (black squares) and after (red squares) spray coating of the polystyrene protecting layer, Fig. S5: detailed photovoltaic parameter evolution as a function of the immersion time in water of covered pure (red squares) and mixed (black squares) perovskite solar cells, Fig. S6: detailed photovoltaic parameter evolution as a function of the light exposure time of covered (squares), pure (red squares) and mixed (black squares) perovskite, and uncovered (circles), pure (black circles) and mixed (black circles) perovskite solar cells. See DOI: https://doi.org/10.1039/d4su00641k |
| ‡ These authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2025 |