 Open Access Article
Open Access ArticlePhyco-synthesized inorganic nanoparticles and their biomedical applications
Nishmitha Ramarajabc,
Gobika Thiripuranathar b,
Sagarika Ekanayake
b,
Sagarika Ekanayake *c,
Keerthi Attanayakeb and
Upul Marapanaa
*c,
Keerthi Attanayakeb and
Upul Marapanaa
aDepartment of Food Science and Technology, University of Sri Jayewardenepura, Nugegoda, Sri Lanka
bCollege of Chemical Sciences, Institute of Chemistry Ceylon, Rajagiriya, Sri Lanka
cDepartment of Biochemistry, University of Sri Jayewardenepura, Nugegoda, Sri Lanka. E-mail: [email protected]
First published on 24th April 2025
Abstract
Marine macroalgae have long been utilized commercially and industrially in food, pharmaceutical, and cosmetic industries. The current trend is to synthesize nanoparticles (NPs) utilizing marine macroalgae as they are a rich source of bioactive compounds. Utilization of marine macroalgae and algal-based polysaccharides is becoming a trendsetter as a simple, cost-effective, sustainable method to synthesize metallic and metallic oxide NPs, thus opening up a new field known as phyco-nanotechnology. Due to safe and biocompatible nature, macroalgae based NPs are investigated for their biological activities such as antibacterial, antifungal, antiviral, anti-inflammatory, anticancer and antioxidant activities. This review focuses on metallic and metallic oxide NPs synthesized from marine macroalgae and their biological activities, with a detailed comparison of how various types of NPs differ in their mechanisms to highlight their distinct effects and potential biomedical applications. Furthermore, current innovations of marine macroalgal polysaccharides such as alginate, fucoidan, and ulvan based NPs as well as their promising opportunities in biomedical applications and therapeutics are also reviewed.
Sustainability spotlightMarine macroalgae with their rich array of bioactive compounds have become a remarkable resource for eco-friendly synthesis of metallic and metallic oxide nanoparticles. This review focuses on the algal derived nanoparticles, which hold greater potential for a vast range of biomedical applications including antibacterial, antifungal, antiviral, anti-inflammatory, anticancer and antioxidant activities. These applications align with the SDG- 3 goal of improving health and well-being by developing sustainable, biocompatible alternatives that could immensely improve public health. Furthermore, focus on sustainable utilization of marine algal resources in high value applications aligns with SDG -14 life below water, highlighting the prospects of marine ecosystems to foster innovative and eco-friendly scientific advancement. |
1. Introduction
Nanotechnology is a novel branch of science that involves the concept of producing 1–100 nm sized particles for a myriad of technological uses. Nanoparticles (NPs) have three layered structures consisting of a core, a shell, and a surface layer. The core is the central part, typically composed of the primary material that gives the NP its fundamental properties (metal/metal oxide). The shell, when present, differs chemically from the core and may provide additional functionality, stability, or controlled release properties of the outermost surface layer which will have the chemical ligand functionalized with small molecules, polymers, or surfactants to enhance biocompatibility, reactivity, or dispersion in a given medium.1The NPs synthesized through physical and chemical techniques give rise to several complications related to the stability, aggregation, control of crystal growth, morphology, and size distribution of NPs. Moreover, the high energy consumption, the requirement for sophisticated instruments, and the use of hazardous chemicals limit the widespread application of physical and chemical techniques for NP synthesis. Therefore, the current focus is on the eco-friendly production of NPs using green chemistry principles,2 otherwise known as the biogenic synthesis approach.
In the biogenic synthesis approach of metal NPs, the precursor metal ions are reduced to their respective metal atoms by reacting with reducing agents sourced from a biological entity. The sequential nucleation of metal atoms converts them to nanoclusters leading to the formation of NPs. Capping agents bind to the NP surfaces during the termination phase, stabilizing the NPs by controlling their size and shape, inhibiting further coalescence, and ensuring the formation of thermodynamically stable NPs with a definite shape.1
The biological entity in the biogenic synthesis of NPs could be plant extracts or bacteria, fungi, viruses, and algae, which transform metallic salts to metallic and metallic oxide NPs by environmentally friendly procedures.3,4 Hence, careful consideration is required when selecting the biological entity (organism) for synthesizing NPs.
Algae are known to be the largest primitive photoautotrophic group of eukaryotes. Unicellular and multi-cellular algae are known as microalgae and macroalgae (seaweeds), respectively. Based on the pigmentation, macro and micro algae are classified into three classes namely brown (phaeophyceae), red (rhodophyceae), and green algae (chlorophyceae).5 Marine algae, similar to their terrestrial counterparts, play vital ecological roles in sustaining aquatic environments. They serve as bioindicators of water quality, contribute to bioaccumulation and bioremediation processes, and generate approximately 80% of the atmospheric oxygen essential for the survival of terrestrial organisms.6 Marine macroalgae, in particular, are an abundant natural source of polysaccharides enriched with various phytonutrients. These polysaccharides are unique due to the availability of sulphate moieties in their structural backbone, resulting in sulphated polysaccharides. This feature imparts a wide range of beneficial properties, such as non-toxicity, biodegradability, and biocompatibility to numerous marine macroalgae.7 The external surface of marine macroalgae is negatively charged, largely due to the cross-linked sulphate groups on the complex polysaccharide molecules. Additionally, the cell walls of marine algae are primarily composed of hemicellulose and cellulose, both carbohydrate macromolecules.8
Marine macroalgae are found to have a wide range of pharmaceutical and biological uses and possess strong antimicrobial activity due to the sulphate moiety.9,10 The exploration of marine macroalgae in NP synthesis has led to the emergence of a new discipline termed phyco-nanotechnology. This review offers a novel perspective by comparatively exploring the modes of synthesis and the effect of metals/metal oxides on bioactivities of nanoencapsulated components of marine macroalgae with emphasis on biomedical applications and future potential.
2. Synthesis of marine algae mediated nanoparticles
Marine macroalgae are regarded as bionanofactories for the facile synthesis of NPs, where both live and dried biomasses are utilized.11 They are immensely suitable for the biosynthesis of NPs due to their rapid growth with an average ten times higher biomass growth than higher plants, and the ability to survive under acidic, alkaline, and saline conditions in a wide range of temperatures.Furthermore, marine algae are rich sources of biologically active primary metabolites such as carbohydrates (mono and polysaccharides), proteins, vitamins, polyunsaturated fatty acids, and numerous secondary metabolites such as carotenoids, flavonoids, terpenoids, other pigments, phenolics, and alkaloids. These bioactive compounds/metabolites are efficient reducing, stabilizing, and capping agents for synthesizing NPs.12 Therefore, it is apparent that the compositional variations of marine macroalgae are influenced by the presence of primary and secondary metabolites, which impart various bioactivities that impact the production of NPs and their activities.
Marine algal polysaccharides have become more significant in recent years due to their bioactivities.13 Numerous therapeutic potentialities, such as antibacterial, antiviral, antifungal, anticancer, anticoagulant, antiproliferative, antioxidant and antitumor activities are exhibited by these polysaccharides.14 Alginates, fucoidans, laminarin, and sulphated polysaccharides are more abundant in brown algae, whereas agar, carrageenans, xylans, and mannans are more abundant in red algae, and xylans, sulphated galactans, and ulvanes are more abundant in green algae.15 Metallic NPs, such as gold (Au), silver (Ag), and metal oxide NPs, including zinc oxide (ZnO), copper oxide (CuO), iron oxide (FeO), titanium oxide (TiO2), etc., are synthesized using a variety of marine macroalgal biomass extracts and marine algal polysaccharides.16–18 Compared to other biosynthetic processes, the time taken by algae to synthesize metallic and metallic oxide NPs is less, as their cell wall is composed of mucilaginous polysaccharides and carboxyl groups, which could absorb/accumulate inorganic metallic ions and remodel them into malleable forms.19
The biogenic synthesis of metallic and metallic oxide NPs lines up with the concept of green chemistry, making it an environmentally friendly process. This mode of synthesis of NPs has the advantage of using no hazardous solvents or reagents, thereby reducing the production of waste. Furthermore, sustainability is enhanced by using renewable natural resources.20
Marine algal based synthesis of NPs involves the combination of algal extracts and metal ion precursor solutions (Fig. 1). The bioactive compounds in the algal extract facilitate the reduction of metal ions to the zero valent state which occurs in three stages. The first stage is the activation phase where the metal ions are reduced and nucleation begins which is often signaled by a colour change in the solution. During the second phase (growth phase) the nucleated nanoclusters combine together to form NPs with different shapes and sizes. In the termination phase the shape of the NPs is completely defined. In the case of metallic oxide NPs, a base (sodium hydroxide) is added along with the metal ions and algal extract initiates metal hydroxide formation. This precipitate on heating decomposes into the corresponding metal oxide NPs. Several factors including precursor ion concentration, pH, temperature and stirring time play a crucial role in determining the shape and size of the resulting NPs.21
Nanoparticle synthesis using marine macroalgae requires an initial extraction process to isolate bioactive compounds. This extraction could be conducted either intracellularly or extracellularly, utilizing water or organic solvents such as ethanol. This step is crucial for obtaining compounds such as polysaccharides, phenolics, flavonoids, and proteins, which are essential for the reduction and stabilization of NPs.
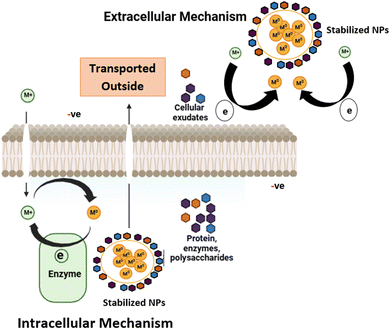
The biosynthesis of algal-derived NPs could be either intracellular or extracellular, depending on where the NPs are formed (Fig. 2). The intracellular method involves biosynthesis of NPs within the algal cells, where the intracellular enzymes (NADPH or NADPH-dependent reductase) and bioactive molecules act as reducing and capping agents. For instance, Ag NPs synthesized using an extract from Padina sp. marine algae prepared through intracellular extraction, facilitated the reduction of silver nitrate (AgNO3) to form Ag NPs.2,17
During extracellular synthesis, metal ions are adhered to the exterior of algal cells and are reduced at the surface by the secreted bioactive compounds such as proteins, lipids, RNA and DNA, which could also act as capping agents. This mode of synthesis is more convenient as NPs are readily purified; however, some pretreatments, such as washing and blending of the algal biomass, are required. For example, extracellular extracts prepared from the brown macroalgae, Sargassum muticum, demonstrated effective capping abilities in synthesizing biocompatible and stable Ag NPs.22
3. Biological activities of marine algal based nanoparticles
3.1 Antibacterial activity
Efficient and safe treatment alternatives for drug-resistant strains are a serious challenge in the field of biomedicine due to the emergence of bacterial resistance to antibiotics. The use of NPs in place of antibacterial treatments is extremely effective in eliminating bacteria and has thus become a popular remedy. NPs have a broad spectrum of antibacterial activity against both Gram-positive and Gram-negative bacteria due to their ability to interfere with the cell membrane and produce reactive oxygen species (ROS) which are detrimental to the survival of bacteria.23 Metal as well as metal oxide NPs synthesized using macroalgae have shown potential for antibacterial activity.3.1.2.1 Silver (Ag) nanoparticles. Silver (Ag) is referred to as being oligodynamic due to its remarkable ability to prevent infections, heal wounds, and reduce inflammation at low concentrations. Recent research has demonstrated that Ag NPs strongly inhibit and act as a protective barrier against the majority of microorganisms, including viruses, fungi, and drug-resistant bacteria.24 Antibacterial activities of Ag NPs are significantly enhanced when combined with antibiotics, while their toxicity to human cells decreases due to the ability to use lower doses.25 Some other metallic NPs such as Cu NPs have a higher ability to inhibit bacterial growth compared to Ag NPs; however, the reaction mechanism damages the bacterial membrane, and they are unstable and hence, easily oxidized into CuO NPs.26 Therefore, more studies have focused on discovering the antibacterial properties of Ag NPs synthesized from marine macroalgae against various bacterial strains.
Ag NPs (85 nm) synthesized from brown marine algae Sargassum polycystum exhibited promising antibacterial activity, which was assessed using the agar well diffusion method. The inhibition zones against Staphylococcus aureus (36 mm) followed by Micrococcus luteus (35 mm), Pseudomonas fluorescens (25 mm), Serratia marcescens, Klebsiella pneumoniae, and Bacillus subtilis (18 mm), indicated the potential of Ag NPs (50 μL) synthesized from S. polycystum as an antibacterial agent.27
Spherical shaped Ag NPs (14 nm) synthesized from another brown marine algae, Padina tetrastromatica, were tested for various pathogenic bacteria using the agar well diffusion method by using varying concentrations of Ag NP solutions (30 μL, 60 μL, and 90 μL). At the highest concentration of Ag NPs (90 μL), B. subtilis showed an inhibition zone of 14 mm, Klebsiella planticola exhibited an inhibition zone of 19 mm, and Pseudomonas aeruginosa demonstrated the highest inhibition zone of 27 mm. Furthermore, Ag NPs (40.45 nm) synthesized from brown marine algae Padina spp. were assessed using the disc diffusion assay. In this study, different concentrations of Ag NP solutions (0.25 mg mL−1, 0.50 mg mL−1, 0.75 mg mL−1, and 1.00 mg mL−1) were evaluated against an ampicillin disc (25 μg) as the positive control. At the highest concentration of Ag NPs (1.00 mg mL−1), S. aureus exhibited the largest zone of inhibition (15.7 mm) followed by B. subtilis (12.67 mm). For the Gram-negative bacteria, P. aeruginosa had the highest zone of inhibition (13.33 mm) while E. coli showed an inhibition zone of 12.67 mm. These results indicate that the Ag NPs demonstrated significant antibacterial activity against both Gram-positive and Gram-negative bacterial strains.17 Accordingly, the ability of brown algae to inhibit bacteria and the positive correlation between the NP concentration and the antibacterial activity are apparent.
When comparing the Ag NPs synthesized from brown algae S. polycystum, P. tetrastromatica, and Padina spp. against the same bacterial strain B. subtilis, the highest inhibition zone (18 mm) was observed with Ag NPs synthesized from S. polycystum demonstrating the significant impact of algal compounds in enhancing antibacterial potency. Smaller Ag NPs (14 nm) synthesized from P. tetrastromatica showed moderate activity (14 mm), proving to be less effective than larger particles synthesized from S. polycystum (85 nm). Furthermore, Ag NPs synthesized using Padina spp. demonstrated the lowest inhibition zone (12.67 mm), indicating that particle size alone does not determine antibacterial efficacy. Other factors such as concentration of bioactive compounds used to synthesize NPs and the number of bioactive compounds remaining on the surface of the NPs, could also play a significant role in determining antibacterial efficacy.
The colloidal Ag NPs (10 nm) synthesized with the green marine algae Caulerpa serrulata extract (20 V/V%) with AgNO3 solution (1 mM) inhibited Escherichia coli with an inhibition zone of 21 mm (for 75 μL of Ag NPs) and Salmonella typhi with an inhibition zone of 10 mm at a lower NP concentration (50 μL of Ag NPs).28
The mechanisms postulated for the antibacterial activity include the adverse effect of NPs on respiration and permeability when attached to the cell membranes of the bacteria. It is proposed that Ag NPs continuously release Ag+ which destruct24 the cell membrane due to the electrostatic attraction between Ag+ and sulphide ions (S2−) of sulphur proteins, in the cell wall and cytoplasmic membrane. The rupture of the bacterial envelope increases the permeability of the cytoplasmic membrane allowing entry of Ag+ into the cell. Once Ag+ enters the cell, the respiratory enzymes are deactivated, producing ROS while interrupting adenosine triphosphate production,29 thereby reducing energy availability for cellular metabolism. ROS have the potential to be a major cause of DNA modification and cell membrane disruption. Due to the fact that phosphorus and sulphur are essential parts of DNA, interactions between Ag+ and these elements disrupt DNA replication, impair cell division, or cause the microorganisms to die. Furthermore, by denaturing ribosomes in the cytoplasm, Ag+ prevents the synthesis of new proteins. Thus, the main causes of antibacterial reactions are bacterial surface adsorption and inhibition of intracellular enzyme activity30 through DNA modification.
3.1.2.2 Gold (Au) nanoparticles. Gold NPs have also garnered significant attention for biomedical applications due to their excellent biocompatibility, low toxicity, ease of surface functionalization, and advantageous optical and electronic properties.31 These NPs are another type of metallic NPs that have been widely produced from marine algal extracts and have demonstrated numerous biological activities including antibacterial activity.32
A portion (20 μL) of Au NPs synthesized using an aqueous extract (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 w/v %) of the red marine algae Gelidiella acerosa was evaluated for antibacterial activity using the agar well diffusion method. The Au NPs demonstrated strong antibacterial effects against E. coli, Serratia marcescens, K. pneumoniae, and B. subtilis. The highest efficacy was observed against B. subtilis (18 mm), followed by E. coli (17 mm), K. pneumoniae (15 mm), and S. marcescens (14 mm).32
20 w/v %) of the red marine algae Gelidiella acerosa was evaluated for antibacterial activity using the agar well diffusion method. The Au NPs demonstrated strong antibacterial effects against E. coli, Serratia marcescens, K. pneumoniae, and B. subtilis. The highest efficacy was observed against B. subtilis (18 mm), followed by E. coli (17 mm), K. pneumoniae (15 mm), and S. marcescens (14 mm).32
The Au NPs synthesized using an aqueous extract (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 (w/v) %) of the brown marine algae Stoechospermum marginatum and AuCl4 solution were tested for antibacterial activity using the agar well diffusion method against various pathogenic Gram-negative and Gram-positive bacteria. The Au NPs demonstrated significant antibacterial activity, particularly against Enterococcus faecalis with an inhibition zone of 11 mm, which was greater than the efficacy of the positive control, tetracycline (0.25 mg mL−1), which exhibited an inhibition zone of 9 mm. In contrast, the smallest inhibition zone of 6 mm was observed against K. pneumoniae, while the NPs showed no inhibitory effect against E. coli. These results highlighted the variable efficacy of the NPs synthesized via the green route using marine algal extracts against both Gram-positive and Gram-negative bacteria.33
500 (w/v) %) of the brown marine algae Stoechospermum marginatum and AuCl4 solution were tested for antibacterial activity using the agar well diffusion method against various pathogenic Gram-negative and Gram-positive bacteria. The Au NPs demonstrated significant antibacterial activity, particularly against Enterococcus faecalis with an inhibition zone of 11 mm, which was greater than the efficacy of the positive control, tetracycline (0.25 mg mL−1), which exhibited an inhibition zone of 9 mm. In contrast, the smallest inhibition zone of 6 mm was observed against K. pneumoniae, while the NPs showed no inhibitory effect against E. coli. These results highlighted the variable efficacy of the NPs synthesized via the green route using marine algal extracts against both Gram-positive and Gram-negative bacteria.33
When comparing the above studies related to Au NPs, red and brown algae exhibited different inhibitory effects with the same strains of bacteria. These differences could be attributed to variations in the concentration of the algal extracts used during synthesis of NPs, as well as the differing types and concentrations of bioactive compounds present in the red and brown algae. Such factors likely influence the synthesis, size, surface characteristics, and overall antimicrobial activity of the Au NPs.
A comparative study on the antibacterial effects of Ag and Au NPs against S. aureus showed that Ag NPs, synthesized using the green method with an average size of 10–20 nm, exhibited a significantly lower Minimum Inhibitory Concentration (MIC) of 4.86 μg mL−1, suggesting higher potency. In contrast, Au NPs synthesized using a similar method but with an average size of approximately 40 nm, required a much higher concentration (197 μg mL−1) to initiate bacterial inhibition.34 The ability to inhibit bacterial growth by Au NPs is relatively less compared to that of Ag NPs as Ag NPs readily release an electron to become Ag+ which enhances the antibacterial activity. Therefore, Au NPs do not show evident inherent antibacterial activities35 as charge is an important factor in interacting with the cell membrane. This highlights the correct choice of metal in synthesizing the NPs for a particular use.
3.1.3.1 Zinc oxide (ZnO) nanoparticles. The wide bandgap, high electron mobility, and transparency in the visible spectrum make ZnO NPs valuable semiconductors. ZnO NPs demonstrate enhanced antimicrobial properties against both Gram-positive and Gram-negative bacteria, as well as uni and multicellular fungi compared to their microscale counterparts. For example, ZnO NPs synthesized using aqueous extracts from Ulva lactuca and S. marginatum showed strong antibacterial activity against S. aureus, E. coli, S. typhi, and Proteus vulgaris. These NPs proved to be more effective than both the metal precursor (ZnSO4) and the algal aqueous extract.36 Furthermore, ZnO NPs synthesized from an aqueous extract of green marine macroalgae Ulva fasciata Delile exhibited strong antibacterial activity against both Gram-positive and Gram-negative bacteria. The results revealed that the antibacterial effect was directly proportional to the concentration of ZnO NPs. The highest antibacterial activity was recorded at a concentration of 200 μg mL−1, particularly against Pseudomonas aeruginosa, followed by E. coli, B. subtilis, and S. aureus, with inhibition zones of 21.7 ± 0.6 mm, 18.7 ± 0.6 mm, 14.7 ± 0.6 mm, and 14.7 ± 1.2 mm, respectively.37
3.1.3.2 Cobalt oxide (Co3O4) nanoparticles. Cobalt oxide (Co3O4) NPs (7.6–28.8 nm) synthesized from marine red algae Grateloupia sparsa demonstrated antibacterial activity against B. subtilis, S. aureus, P. aeruginosa and E. coli. At a dose of 30 mg mL−1, the zone of inhibition of Co3O4 NPs against B. subtilis, S. aureus, P. aeruginosa, and E. coli was 11.7 mm, 12.5 mm, 14.3 mm, and 17.6 mm, respectively.38
Based on the zone of inhibition measurements, it can be concluded that Co3O4 NPs were less effective compared to the effect of ciprofloxacin (zone of inhibition of 18.1 mm for 30 mg mL−1). The mechanism of action of the oxides is attributed to their smaller size and higher surface area to volume ratio, facilitating their interaction with bacterial cell membranes and increasing permeability. ROS penetration into the cytoplasm causes nuclear and plasmid damage, resulting in a change in cell signaling, finally leading to cell death.
3.1.3.3 Copper oxide (CuO) nanoparticles. Copper oxide (CuO) NPs are distinguished by their antimicrobial properties and their ability to navigate biological barriers effectively, allowing them to reach target organs based on their size and surface characteristics.39
CuO NPs (83 nm) synthesized from brown marine algae Padina boergesenii showed antibacterial activity against pathogenic bacteria E. coli, P. aeruginosa, and B. subtilis in a study with ampicillin as the control. At a concentration of 200 μg mL−1, CuO NPs were able to significantly inhibit pathogens (inhibition zone of E. coli – 15 mm, P. aeruginosa – 11 mm, and B. subtilis – 21 mm).40
CuO NPs of 5–45 nm, biosynthesized from the brown algal extract of Bifurcaria bifurcate, when tested for antibacterial activity against Enterobacter aerogenes and Staphylococcus aureus using the disc diffusion method exhibited inhibition zones of 14 mm and 16 mm, respectively. The B. bifurcata extract contains compounds such as diterpenoids which can fulfill dual roles in reducing and stabilizing CuO NPs. The discs containing algal extract alone displayed no zone of inhibition, indicating that at the concentration employed for synthesizing CuO NPs, the algal extract did not exhibit any antibacterial activity. However, CuO NPs synthesized from the extract demonstrated notable antibacterial activity. This effect could be attributed to the significantly large surface area of CuO NPs and the presence of higher levels of amines and carboxyl groups on the cell surface of the microorganisms, resulting in enhanced affinity of Cu2+ towards these groups. Subsequently released Cu2+ due to surface oxidation of CuO NPs have the potential to bind with DNA molecules, leading to disruption of the helical structure through cross-linking within and between nucleic acid strands.41
When comparing the antibacterial effect of similar concentrations of metal oxide NPs such as CuO (83 nm) and Co3O4 NPs (7.6–28.8 nm) on B. subtilis, Co3O4 NPs demonstrate higher activity than CuO NPs. This may be due to the smaller size and higher surface area to volume ratio of Co3O4 NPs compared to CuO NPs.
3.2 Antifungal activity
Resistance of pathogenic fungi to currently available antifungals has become a worldwide epidemic. Compared to bacterial infections, the medications for invasive fungal infections are quite limited. For example, exposure to fungal agents worsens bronchial asthma, which is a serious public health concern.42 Some bioactive compounds present in the marine macroalgae Acanthaophora specifera, Cladophoropsis sp., and Laurencia paniculata tested for bronchial asthmatic pathogens demonstrated excellent inhibitory activities.43 An antifungal study conducted against the dermatophytes Trichophyton mentagrophytes and Microsporum canis using the methanolic extract of Gracilaria corticata demonstrated significant antifungal activity.44 Although there has been considerable research on the antifungal activity of marine algal extracts, relatively few studies have investigated the antifungal properties of NPs synthesized from marine macroalgae.To mitigate decay caused by pathogenic organisms, numerous studies have examined the effectiveness of NPs in preserving food products, particularly fruits. A study investigated the antifungal activity of biogenic Ag NPs synthesized from the aqueous extract of the brown marine algae Turbinaria turbinata, examining their effectiveness as a coating on tomato fruits to protect against Penicillium italicum (OR770486) during a 17 day storage period. Ag NP (spherical shaped of size 14.5–38.9 nm) coated tomato samples retained their appealing appearance throughout the above storage period. The use of Ag NP coating enhanced the shelf life of the tomatoes by maintaining their quality and delaying fungal decay.45 However, acute and chronic toxicity studies are needed prior to these being approved for use as cellular penetration is a possibility.
A broad range of diseases from cutaneous mucositis to invasive conditions such as hepatosplenica candidiasis, peritonitis, and systemic candidiasis are caused by Candida spp. Treatments with antifungal activity are necessary due to serious health problems, such as renal and liver malfunction caused by infectious Candida spp.46 Spherical Ag NPs with an average size of 51.82 nm synthesized from marine macroalgae G. corticata had potent antifungal activity against Candida spp. It was revealed that 30 μL of NP solution impeded Candida albicans (inhibition zone of 12 mm) and Candida glabrata (inhibition zone of 11 mm) growth. However, the biosynthesized Ag NPs were not as effective as nystatin which was the control (inhibition zone of 17 mm and 19 mm for C. albicans and C. glabrata respectively). Moreover, it was observed that Ag NPs synthesized from Hypnea muciformis demonstrated higher antifungal efficacy than the H. muciformis extract and 1 mM AgNO3 solution alone.47 Thus, this finding suggests the ability of algae to influence the antifungal effects of Ag NPs.
Silver NPs stimulate the production of hydroxyl radicals, which in turn cause C. albicans cell destruction. Each time fungal cells are exposed to Ag NPs, 'pits' grow on the membrane's surface, destroying the integrity of the cells.29 It is also found that the size of the NPs affects the antifungal activity against Candida spp. For example, smaller Au NPs (9 nm) have exceptional antifungal activity compared to larger Au NPs (13 nm).30 Thus, it is suggested that smaller NPs exhibit better antifungal activity similar to that observed with antibacterial activity.
Magnesium oxide (MgO) NPs synthesized using an aqueous algal extract of brown marine algae Sargassum wightiii demonstrated antifungal activity against Aspergillus fumigates, Fusarium solani and Aspergillus niger. These biosynthesized MgO NPs were shown to be more potent when compared to the positive control, fluconazole. Among the three strains tested, MgO NPs inhibited the growth of F. solani and A. niger more effectively than A. fumigatus.48
The antifungal activity of MgO NPs is attributed to various mechanisms. First, it could involve electrostatic interactions between the phosphate groups in the cell membrane and Mg2+ present on the surface of MgO NPs. This interaction makes it easier for the MgO NPs to enter the fungal cell. Mg2+ may then attach to the thiol groups of the cell's proteins, causing denaturation of the proteins and interference with cellular processes. Additionally, MgO NPs may cause cellular death by producing ROS through oxidative stress mechanisms. ROS cause damage to lipids, proteins, and DNA, which eventually results in cell death. Overall, electrostatic interactions, protein denaturation, and ROS mediated oxidative stress are probably all involved in the antifungal activity of MgO NPs. These factors collectively contribute to inhibition of fungal growth, which may explain the higher activity of MgO NPs compared to the control.49
3.3 Anticancer activity
It has been reported that a variety of compounds such as fucoidans, laminarian, and terpenoids from marine macroalgae posess anticancer and antiproliferative qualities.50 Thus, the biosynthesized NPs from marine algae have gained popularity as potential therapeutics for cancer as well. Since NPs could be coupled with a variety of ligands, RNA, DNA, aptamers, peptides, and antibodies, the modified NPs facilitate drug transportation to the action site, improving both its pharmacokinetic properties and therapeutic efficacy against cancer. The increasing global prevalence of cancer makes it inevitable that novel anticancer drugs will be sought after51,52 with nanotechnology based anticancer treatment modalities taking precedence.Copper oxide NPs mediated by the marine red algae Pterocladia capillacea were employed to enhance the anticancer activity of nedaplatin, an anticancer drug. These CuO NPs (62 nm) loaded with 0.09 mM nedaplatin revealed a sustained release of the drug, reaching the maximum at 120 hours. Cytotoxicity assays conducted on various cell lines, including hepatocellular carcinoma (HEP-G2), breast cancer (MCF-7), and ovarian cancer (SKOV-3), resulted in IC50 values of 0.40 ± 0.08, 1.50 ± 0.12, and 0.70 ± 0.09 μg mL−1, respectively. The results showed a higher rate of cell death for the nedaplatin loaded NPs when compared to free nedaplatin (IC50 = 14.5 ± 1.73 μg mL−1). This work highlights the potential of biologically synthesized metal NPs from algal extracts, which could be influential in the development of other metallic NPs for various applications, inlcuding anticancer drug delivery.53
Silver NPs (10 nm) synthesized from brown marine algae Sargassum vulgare exhibited anticancer activity against HeLa cells and human myeloblastic leukemic cells while Ag NPs from S. muticum showed cytotoxic activity against the MCF-7 breast cancer cell line. These Ag NPs influenced the production of ROS through an intracellular pathway. When Ag NPs were internalized, the ROS levels within the cancer cells significantly increased. The elevated levels of ROS in turn caused oxidative stress eventually damaging cellular macromolecules like DNA, proteins, and lipids. This oxidative damage disrupts cellular function and integrity, resulting in apoptosis and eventual death of cancer cells.2
Human breast adenocarcinoma cell lines (MCF-7) and skin normal cell lines (HFb-4) were used to test the anticancer potential of Au NPs produced using Ulva rigida, Cystoseira myrica, and Gracilaria foliifera. At a concentration of 188 μg mL−1, the Au NPs produced from G. foliifera (red algae) showed the strongest anticancer potential (92.13%), followed by C. myrica (89.82%) and U. rigida (86.83%) in MCF-7 cells, respectively.36
The cytotoxicity of Au NPs synthesized using U. rigida, C. myrica, and G. foliifera was evaluated for their anticancer potential in both skin normal cell lines (HFb-4) and human breast adenocarcinoma cell lines (MCF-7). Among them, the Au NPs synthesized from G. foliifera (red algae) demonstrated the highest anticancer potential (92.13%), followed by C. myrica (89.82%) and U. rigida (86.83%) in MCF-7 cells, respectively, at the same concentration (188 μg mL−1).36
The anticancer efficacy of Au NPs synthesized from the aqueous extract of the red marine algae Acanthophora spicifera was evaluated by assessing cell viability using the MTT assay. When tested against HT-29 cells, the Au NPs (20 nm) exhibited a low IC50 value of 21.86 μg mL−1, indicating their strong anticancer activity. The cytomorphological alterations of healthy polygonal shaped cells to shrunk altered structures validated the anticancer potential of biosynthesized Au NPs.54 Thus, in contrast to lower antibacterial activity of Au NPs, a higher anticancer potential is demonstrated. The smaller size and the functional groups available in red algae could have contributed to the higher anticancer activity.35
Furthermore, NPs loaded with the bioactive compound fucoidan extracted from brown marine algae Cladosiphon okamuranus against osteosarcoma cells using liposomes as nanocarriers induced apoptosis compared to native fucoidan, with a maximum reduction of 80% in cell viability (2 mg mL−1).55 Native fucoidan extracted from C. okamuranus has a molecular weight of 80 kDa. To enhance its bioactivity, especially its anticancer effects, native fucoidan was hydrolyzed to produce low molecular weight fucoidan which has a molecular weight range of 2–10 kDa. The cytotoxic effects of native fucoidan and fucoidan lipid NPs were compared on osteosarcoma in vitro and in vivo aiming to analyze the size dependent bioactivities of fucoidan.54 Thus, the size and molecular weight of fucoidan directly influence its biological effects, particularly in the context of anticancer activities, as well as its potential for systemic delivery using NPs.
3.4 Antiviral activity
According to recent reports, viruses cause approximately 2 million deaths worldwide each year. So far, vaccination is the most effective approach in preventing viral infections. Unfortunately, the number of effective vaccines against viruses is relatively less. Among the numerous potential antiviral drugs, nanotechnology has demonstrated potential in this field.56All viruses pass through 6 stages during their life cycle i.e. attachment to host cells, penetration, uncoating, replication, assembly, and release to target cells.57 Marine polysaccharides contribute to antiviral activities in 2 ways: (1) impede the activity of the virus and (2) enhance the host's immunological response against the virus. According to their structure, chemical makeup, and preferred mode of antiviral activity, antiviral polysaccharides have the potential to block viral infection at any of the six main stages of the life cycle of the virus. In several antiviral reactions, polysaccharides have been shown to have a direct virucidal effect, in which the binding of the polysaccharides inactivates the virus itself. Another antiviral mechanism is the inhibition of viral adsorption, which is adapted by marine algal polysaccharides such as galactans, carrageenans, and fucoidans.58 Numerous sulphated marine polysaccharides, such as iota-carrageenan from red algae and fucoidan from brown algae, have demonstrated inhibitory action against SARS-CoV-2 as well.57
The family Flaviviridae contains at least 72 different viruses, including West Nile, Yellow fever, and Japanese encephalitis viruses. The Aedes aegypti and Aedes albopictus mosquitoes are the primary vectors of the enclosed, single-stranded, positive-sense RNA dengue virus.59 The antiviral activities of metal and metal oxide NPs such as Ag, Au, TiO2, and SiO2 against several viruses such as dengue virus type-2, hepatitis B virus, HIV-1, influenza virus, foot and mouth disease virus H3N2 and H1N1 are reported. Ag NPs attach to the envelope proteins of dengue virus type-2, blocking the virus from binding to the host cells. This inhibition at the entry stage prevents viral replication within the host cell. Au NPs are often functionalized with anti-HIV drugs or molecules to enhance their specificity and antiviral effects. Functionalized Au NPs block the virus from binding to host cells or inhibit reverse transcriptase, an enzyme essential for viral replication. TiO2 NPs, when exposed to UV light, generate ROS that damage the viral lipid envelope and proteins, leading to the inactivation of both H3N2 and H1N1 influenza strains.60 Fucoidan extracted from brown marine algae Solanum marginatum containing high sulphate content used in synthesizing ZnO NPs has shown to be 99.09% effective against dengue virus type-2.61
It was postulated that selenium (Se) NPs possess viral-prevention capabilities due to their ability to interfere with viral replication and to modulate immune responses, as well as their oxidative properties that disrupt viral structures. However, Se NPs synthesized from brown marine algae Polyladia myrica were examined for antiviral properties against herpes simplex (HSV-2), hepatitis A (HAV), and adenovirus using MTT assay. The Vero cell line demonstrated 40.25% antiviral activity against the HAV virus but lower antiviral activities against adenovirus (8.64%) and HSV-2 (17.39%).62
3.5 Anti-inflammatory activity
Inflammation is a nonspecific defensive reaction to unfavorable stimuli such as pathogens, toxic chemicals, and specific tissue damage. The main purpose of inflammation is to safeguard tissues from aforesaid conditions by eliminating the cause of inflammation and to revitalize tissue repair mechanisms. Multiple sclerosis, cancer, inflammatory arthritis, atherosclerosis, coronary artery disease, obesity, dermatitis, migraines, interstitial cystitis, irritable bowel syndrome, insulin resistance, and a variety of other disease conditions could be caused and aggravated by the effects of inflammation, particularly chronic conditions.63As mentioned earlier, marine macroalgae contain sulphated polysaccharides, polyphenols, tannins, fatty acids, and proteins possessing anti-inflammatory properties among other bioactivities. Consequently, they have become a significant focus in medicinal research, offering protective effects against inflammatory diseases and potentially replacing synthetic drugs currently in use. Particularly, phlorotannins from brown marine algae have been found to be potent inhibitors of pro-inflammatory cytokines such as inducible nitric oxide synthase, cyclooxygenase-2, tumor necrosis factor- I alpha, interleukin-1 beta and interleukin-6.64
Anti-inflammatory activity of biosynthesized Se NPs from marine macro brown algae Polycladia crinite was evaluated using the carrageenan induced rat paw edema model. The percentages of edema inhibition in pretreated groups with doses of 25 and 50 mg kg−1 were 62.78% and 77.24%, respectively. These results implied that Se NPs from P. crinite are a potent anti-inflammatory agent and the activity is concentration dependent.65
Proteinase inhibitors offer remarkable protection against inflammatory reactions due to tissue damage caused by leukocyte proteinase. Ag NPs synthesized from marine macroalgae Galaxaura elongate (red), Turbinaria ornata (brown), and Enteromorpha flexuosa (green) exhibit strong anti-inflammatory properties due to their antiproteinase activity. Furthermore, proteinase inhibitory activity showed the highest inhibitions of 59.78%, 44.40%, and 47.38% at 200 μg mL−1 of Ag NPs derived from G. elongate, T. ornata, and E. flexuosa, respectively, in comparison to 64.28% inhibition of diclofenac sodium (control) at the same concentration (200 μg mL−1),66 suggesting the potential of these algal derived NPs in developing anti-inflammatory drugs. This finding suggests that red algae derived Ag NPs hold the most promising anti-inflammatory properties compared to those derived from brown and green algae.
3.6 Antioxidant activity
Chemical entities with one or more unpaired electrons in their outermost shell are known as free radicals. They seek and capture electrons from other substances to become neutral and stable. Although the initial reaction neutralizes one free radical, it could lead to the production of another free radical, initiating a chain reaction that ultimately results in the formation of ROS. ROS include hydrogen peroxide and hydroxyl ions which are highly reactive and cause damage to proteins, DNA, and lipids by amending the biochemical compounds, eventually affecting the cell. These molecular changes contribute to diseases such as cancer, coronary heart disease, inflammatory diseases, neurological degeneration, atherosclerosis, and respiratory issues.67 Thus, as the first line of defense against most diseases, antioxidants play a crucial role. Therefore, for counteracting the deleterious effects of free radicals, compounds with antioxidant activities are crucial.Among various bioactive compounds identified from marine resources, 8000 different compounds were found to be polyphenols, which display significant antioxidant properties. Specifically brown marine algae are rich in polyphenolic compounds ranging from simple phenolic acids to complex phlorotannins.68 Thus, these marine macroalgae are extensively investigated for bioactive compounds with antioxidant activities.
Cerium oxide (CeO2) NPs synthesized from methanolic extracts of brown marine algae Sargassum wightii Greville demonstrated an increased percentage of radical scavenging activity with increasing concentrations of NPs. Thus, 100 μg mL−1 of CeO2 NP solution demonstrated the highest antioxidant activity (86.4%) which was comparable to that of standard vitamin C (92.6%) of the same concentration67 when assessed with DPPH assay (2,2-diphenyl-1-picrylhydrazyl).
The antioxidant capacity of biosynthesized Co3O4 NPs from red marine algae Grateloupia sparsa demonstrated the highest DPPH radical scavenging activity (88.2%) at 500 μg mL−1. It is assumed that Co3O4 NPs act as electron donors interacting with free radicals to morph them into stable molecules and terminate the radical chain reaction38 thus preventing cellular damage.
The radical scavenging activity of the Ecklonia cava extract and biosynthesized Ag NPs (43 nm) using the E. cava extract was similar at the same concentrations (100, 250, and 500 μg mL−1) when assayed with DPPH. This high comparative antioxidant activity of Ag NPs was attributed to the E. cava extract remaining on the surface of Ag NPs. Due to the effective antioxidant properties of both E. cava extracts69 and Ag NPs, a combination of Ag NPs and E. cava extract with collaborative effects could be a promising candidate for pharmaceutical and nutraceutical products.70 Tables 1 and 2 present a comprehensive overview of phyco-synthesized NPs, highlighting their biological activities and corresponding applications.
| Marine macroalgae | NP | Size of NPs | Biological activity | Application | Reference |
|---|---|---|---|---|---|
| Metal NPs | |||||
| Padina pavonica | Ag | 20–70 nm | Antibacterial activity | Antibacterial agent against S. aureus, B. subtilis, E. coli and P. aeruginos | 71 |
| Sargassum swartzii | Ag | 14–30 nm | Antibacterial activity | Antibacterial agent against B. subtilis and S. aureus | 72 |
| Sargassum vulgare | Ag | 6.90–16.97 nm | Antibacterial activity | Antibacterial agent against Bacillus mojavensis, Staphylococcus caprae, Staphylococcus capitis, and Staphylococcus epidermidis | 73 |
| C. serrulata | Ag | 10 nm | Antibacterial activity | Antibacterial agent against S. aureus, P. aeruginosa, Shigella sp., S. typhi, and E. coli | 28 |
| Padina sp | Ag | 25–60 nm | Antibacterial activity | Antibacterial agent against S. aureus and P. aeruginosa | 17 |
| Cystoseira baccata | Ag | 22 nm | Antibacterial activity | Antibiofilm agent against, P. aeruginosa and E. coli | 74 |
| Turbinaria conoides | Ag | 60 nm | Antibacterial activity | Antibacterial agent against Streptococcus sp., B. subtilis and K. pneumoniae | 75 |
| Gelidiella acerosa | Ag | 59 nm | Antibacterial activity | Antibacterial agent against B. subtilis | 76 |
| Padina tetrastromatica | Ag | 14 nm | Antibacterial activity | Antibacterial agent against B. subtilis, K. planticola, and P. aeruginosa | 77 |
| Stoechospermum marginatum | Au | 18.7–93.7 nm | Antibacterial activity | Antibacterial agent against P. aeruginosa, Klebsiella oxytoca, E. faecalis, K. pneumoniae, Vibrio parahaemolyticus, Vibrio cholerae, E. coli, S. typhii, Salmonella paratyphi, andP. vulgaris | 33 |
| Gracilaria sp | Ag–Au | 22–30 nm | Antibacterial activity | Antibacterial agent against S. aureus, E. coli, K. pneumoniae, S. typhii and P. aeruginosa | 78 |
| Gracilaria corticata | Ag | 20–55 nm | Anticancer activity | Anticancer agent against the human hepatic carcinoma (HepG2) cell line | 79 |
| Sargassum vulgare | Ag | 10 nm | Anticancer activity | Anticancer agent against HeLa cells and human myeloblastic leukemic cells | 2 |
| Ulva rigida | Ag | 12 nm | Anticancer activity | Anticancer agent against the MCF-7 cell line | 80 |
| Sargassum longifolium | Au | 10–60 nm | Anticancer activity | Anticancer agent against MG-63 human osteosarcoma cells | 81 |
| Padina gymnospora | Au | 14 nm | Anticancer activity | Dose-dependent cytotoxic potential against the human liver cancer cell line (HepG2) and human lung cancer cell line (A549) | 82 |
| Chaetomorpha linum | Ag | 35 nm | Anticancer activity | Anticancer agent against colon cancer cells HCT-116 in vitro | 83 |
| Gracilaria foliifera | Au | 13 nm | Anticancer activity | Anticancer agent against human breast adenocarcinamo cell lines (MCF-7) | 84 |
| Acanthophora spicifera | Au | <20 nm | Anticancer activity | Anticancer agent against human colon adenocarcinoma (HT-29) cells | 54 |
| Ulva fasciata | Ag | 8–16 nm | Antitumor activity | Cytotoxic effect against Ehrlich ascites carcinoma (EAC) in vitro | 85 |
| Turbinaria turbinata | Ag | 8–16 nm | Antitumor activity | Cytotoxic effect against Ehrlich ascites carcinoma (EAC) in vitro | 85 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
| Metal oxide NPs | |||||
| Ulva fasciata | ZnO | 3–33 nm | Antibacterial activity | Antibacterial agent against, P. aeruginosa, E. coli, B. subtilis, and S. aureus | 37 |
| Grateloupia sparsa | Co3O4 | 7.6–28.8 nm | Antibacterial activity | Antibacterial agent against B. subtilis, S. aureus, P. aeruginosa and E. coli | 38 |
| Padina boergesenii | CuO | 83 nm | Antibacterial activity | Antibacterial agent against E. coli (MTCC 443), P. aeruginosa (MTCC 424), and B. subtilis (MTCC 5981) | 40 |
| Bifurcaria bifurcata | CuO | 5–45 nm | Antibacterial activity | E. aerogenes and S. aureus | 40 |
| Padina boergesenii | CuO | 76 nm | Anticancer activity | Anticancer agent against human malignant melanoma (A375 cell line) | 40 |
| Marine macroalgae | NP | Size of NPs | Biological activity | Application | Reference |
|---|---|---|---|---|---|
| Metal NPs | |||||
| Gracilaria corticata | Ag | 51.82 nm | Antifungal activity | Antifungal agent against Candida spp. | 47 |
| Polycladia crinite | Se | Anti-inflammatory activity | Anti-inflammatory agent against the edema rat paw model | 65 | |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
| Metal oxide NPs | |||||
| Sargassum wightii | MgO | 68.06 nm | Antifungal activity | Antifungal agent against A. fumigates, F. solani and A. niger | 48 |
| Sargassum spp. | ZnO | 20–200 nm | Anti-inflammatory activity | Anti-inflammatory activity against arthritis disease | 86 |
| Sargassum wightii Greville | CeO2 | 35–68 nm | Antioxidant activity | Free radical scavenging activity with an IC50 of 0.99 | 67 |
4. Phyco-synthesized nanoparticles in biomedical applications
Nanoparticles, due to their unique physicochemical and optoelectronic properties, have drawn increasing interest in biomedical and pharmaceutical applications. Due to some exceptional characteristics, such as an enhanced targeting system, inertness, and the possibility of its surface being functionalized due to the negative charge, NPs are extensively researched for biomedical applications (Fig. 3).874.1 Targeted drug delivery
The field of nanotechnology has experienced remarkable advancements in the development of nanosized materials for targeted drug delivery. Targeted drug delivery is a significant biomedical application used to deliver drugs to the precise location of the tumor without affecting the neighboring healthy cells in contrast to the conventional drugs currently being used. Understanding the interactions at the interface between NPs and biological components is crucial for optimizing therapeutic efficacy. The most crucial factor in drug delivery systems is pairing a suitable carrier with one or more medicines. Directing the active ingredient to the site of action and delivering the correct dosage for the required duration are the two major requirements for ideal delivery systems.88Due to their nanoscale dimensions, these particles efficiently penetrate tissue systems, facilitating cellular uptake and enhancing drug bioavailability, thereby enabling effective drug delivery.89 Furthermore, NPs circumvent multidrug resistance mechanisms and maintain stability within the blood vascular system until they reach their intended targets, providing a strategic advantage in cancer therapy and other applications.90
When NPs with specific shapes, sizes, and charges are introduced into the body, they are surrounded by active biological components, forming a NP-protein corona complex. This complex provides the NPs with a new biological identity, making specific receptors provided by the protein corona available, which enables them to access target spots sparing the normal cells.91
Recently, special attention has been drawn towards developing drug delivery systems using polysaccharides from marine macroalgal species due to their antibacterial, antiviral, antifungal, and antitumor activities and nontoxic nature compared to synthetic medications.92 The combination of bioactive compounds of macroalgae and NPs may increase therapeutic efficacy while decreasing the eventual toxicity of the transported substances with focused delivery.
Ulvan is an anionic sulfated polysaccharide derived from green macroalgae U. lactua, which consists of glucuronic acid, sulfate, rhamnose, and iduronic acid, possessing significant antioxidant, antitumor, and anticoagulant activities. Ulvan loaded onto chitosan functionalized graphene oxide (GO–CH) NPs creates a novel D-mannose mediated targeted drug delivery system for glioblastoma cancer.96 Graphene oxide (GO), a 2-D nanomaterial made of graphite, is a prospective drug transporter with a larger surface area. As a result, multiple drugs can be delivered from the point of administration to the target site. They possess good water dispersibility and hydrophilicity due to the buildup of hydroxyl, carbonyl, and epoxy groups on the surface.97 The drug release from the mannose decorated chitosan functionalized graphene oxide (GO–CH–Ma) nano-carrier exhibited a more promising profile as the entire nanocarrier system demonstrated pH-dependent ulvan release behavior. The drug carrier's release mechanism is pH-responsive to optimize the delivery of anticancer drugs within tumor tissue while ensuring minimal release during circulation in the bloodstream. Experimental conditions, including pH levels (7.4 and 5.3) and temperature (37 °C), were chosen to mimic physiological conditions accurately. Fitting the release data with drug kinetic models revealed that the release of ulvan from GO–CH follows an anomalous diffusion mechanism.96
Alginate is a polysaccharide extracted from brown marine algae, made up of D-mannuronic acid and L-guluronic acid which has many unique characteristics that have made it possible to be employed as a matrix for the capture and delivery of biological agents. Proteins, cells, and DNA could be integrated into alginate matrices while retaining their full biological activity.98 Alginate magnetic NPs were synthesized by homogenization followed by reticulation with Ca2+. Thus, the produced NPs are highly stable, have excellent magnetic properties, and are able to entrap and release the drug to the target site.99
Physicochemically stabilized bioconjugated Au NPs synthesized from marine macroalgae Padina gymnospora were used as a drug delivery system for controlled cancer therapy. These drug delivery systems are developed in accordance with their ability to distinguish between cancerous and non-cancerous cells, making them a possible replacement for current medications. It is assumed that OH groups present in fucoxanthin, a carotenoid found in the cell walls of algal species might be adsorbed onto the surface of metallic NPs, and thus the ability to stimulate or suppress the immune system has a synergic effect on the anti-proliferative activities.100
Chitosan/fucoidan-taurine conjugated NPs were synthesized for the delivery of berberine for treating the defective intestinal epithelial tight junction barrier caused by bacterial endotoxins. Fucoidan has negatively charged sulphates and carboxyl groups. Fucoidan and chitosan, which have opposite charges, combine electrostatically to generate stable colloidal NPs. These NPs could effectively deliver berberine to epithelial cells without impairing the function of the intestinal barrier thus delivering to the target.101
4.2 Biosensing
Nanoparticles are best suited for building novel and enhanced sensing devices, particularly electrochemical sensors and biosensors, due to their distinctive physicochemical and optoelectrical features. Numerous types of NPs, including metal, metal oxide, and semiconductor NPs, have been utilized to make electrochemical sensors and biosensors, and these NPs have a variety of functions in various sensing systems. Biomolecules are immobilized due to the large surface area and high surface free energy of NPs. Furthermore, these NPs could catalyse electrochemical reactions, act as reactants in electrochemical reactions, transport electrons between electrode surfaces, improve proteins and label biomolecules.102Metal NPs have been widely exploited as biosensors and MRI tracers, and for cell targeting treatments. Particularly, localized surface plasmon resonance (LSPR) is responsible for the colors or emissions of NPs that are identified in the UV-visible region. Biosynthesized Ag NPs using the algal extract of Noctiluca scintillans have been evaluated as a colorimetric sensor for the detection of H2O2 with colour changing from brown to colorless when NPs reacted with H2O2.2
4.3 Safety and toxicological considerations and regulatory aspects of nanoparticles in biomedical applications
The interactions between NPs and biological systems play a crucial role in determining the safety and effectiveness of nanomaterials in biomedicine. Ensuring safety is paramount for regulatory approval and remains a significant challenge across the field of nanomedicine.103 The concern is especially about inorganic NPs which tend to accumulate in the body due to slow degradation and excretion which may lead to long term effects.104The biological effects and potential toxicity of NPs are influenced by intrinsic properties which include physicochemical properties, such as their size, shape, chemical composition, surface characteristics, and aggregation state. Extrinsic properties, such as dosage, cell type, and the surrounding biological environment, also play a vital role in determining their impact.105
The biological impact of algae mediated NPs is often dose-dependent. At lower concentrations, they may exhibit therapeutic effects, while higher doses could cause cytotoxicity.106,107 Understanding the underlying mechanisms of this toxicity remains complicated. For example, there is an ongoing discussion whether the toxicity observed in biological systems is basically caused by Ag NPs themselves or by the Ag+ they release. Ag+ is generated through surface oxidation of Ag NPs and subsequently interacts with biological molecules. Substantial evidence supports the view that the toxic effects are largely due to Ag+ rather than the NPs directly.108 Recent studies have demonstrated that even minimal release of Ag+ from Ag NPs can lead to cytotoxicity.109 Therefore, the nature of Ag NPs is both beneficial and potentially harmful. This highlights the importance of carefully controlling their concentration as well as their intrinsic and extrinsic properties.
Surface coatings and functionalization are applied to NPs to modify and enhance their physicochemical properties. These surface coatings improve stability, alter wettability and dissolution and impart specific functionalities. Moreover, surface modification significantly influences toxicity where harmful particles may be rendered less toxic; otherwise, these particles become more bioavailable and potentially more harmful to human cells. For instance, an in vitro study evaluating the toxicity of silica-coated Fe3O4 NPs on HeLa and A549 cells revealed that surface passivation helps reduce disruptions in iron homeostasis and oxidative stress. Consequently, passivated NPs exhibit lower toxicity during cellular internalization compared to their uncoated counterparts. Thus, surface modification not only serves functional purposes but also forms a critical component in reducing the toxicity of NPs intended for biomedical applications.
5. Limitations
The limitations in synthesizing marine algae mediated NPs include colloidal stability due to a high level of agglomeration over time. While marine algae-derived NPs can be modified with different biomolecules to improve their ability to target specific cells, deliver drugs, or perform as sensors, the process of modification is often complicated and not always consistent.Marine algae, being part of the marine environment, accumulate heavy metals which can extend to algae mediated NPs. Furthermore, limited knowledge regarding the exact mechanism of algae mediated inorganic NP synthesis has restricted the usage of marine algae in NP synthesis.
Although marine algae are rich in bioactive compounds and offer a diverse range of active molecules, extensive research is still needed to fully understand the role of these biomolecules as reducing and capping agents in the synthesis of NPs. Furthermore, more research is required regarding the large-scale production of NPs. Stable, purified marine macroalgae-based inorganic NPs could be synthesized using new emerging characterization technologies for industrial applications. Although there are proven studies with regard to their potential applications in biomedicine, there is much still room for understanding the toxicity and exact effect of NPs in the human body over time with clinical trials.
6. Future perspectives
It is important to consider the limitations encountered thus far and explore future perspectives. For instance, the potential of algal-derived NPs in biofilms (microorganisms attached to surfaces and encased in polymeric substances) can be engineered to serve as carriers for controlled drug delivery systems. Thus, algal derived NPs in biofilm application have not been properly recognized, and remain underutilized. Microalgae based NPs have dominated the biosensing field, but macroalgae based NPs hold untapped potential for future exploration, especially in sustainable biosensing technologies.Marine algae-derived NPs, such as Ag NPs, exhibit multifunctionality (antibacterial and antifungal properties), making these valuable in treating infections caused by both bacteria and fungi. Their dual bioactivity holds great potential for use in biomedical applications offering a more efficient and cost-effective approach to it in the future.
However, only Ag and Au NPs synthesized from marine macroalgae are largely studied to date and the research on other metal and metal oxide NPs remains largely unexplored. Thus, there are unique opportunities for exploration and developing new phyco-mediated synthesis of metal and metal oxide NPs such as palladium (Pd), platinum (Pt), copper (Cu), ZnO, MgO, CuO, etc. for biomedical applications.
Even though significant progress has been made, further research is required to realize the full potential of marine algae and marine algal polysaccharide derived NPs in biomedical applications.
7. Conclusions
Biogenic, facile NPs synthesized from marine macroalgae offer indefinite opportunities in biomedical applications due to the availability of numerous superior bioactive compounds. With the concept of utilizing marine macroalgal extracts as reducing and capping agents, various inorganic NPs have been synthesized in the recent past. As NPs synthesized from marine algae have demonstrated antibacterial, antiviral, antifungal, anti-inflammatory, anticancer, and antioxidant activities, these have high potential in biomedical applications, including as antimicrobial agents, in targeted drug delivery, and for biosensing. Moreover, NPs synthesized from marine algal polysaccharides which are abundant and nontoxic also play a crucial role in biomedical applications, such as targeted drug delivery. While much progress has been made in the synthesis and application of marine algae-derived NPs in biomedicine, there is still more to explore and develop. Continued research efforts are required to pave the way for new discoveries and innovations in this field to further expand the potential of marine algae-derived NPs in a plethora of biomedical applications.Abbreviations
| Ag | Silver |
| AgNO3 | Silver nitrate |
| Au | Gold |
| CeO2 | Ceric oxide |
| Co3O4 | Cobalt oxide |
| CTAB | Cetyltrimethylammonium bromide |
| Cu | Copper |
| CuO | Copper oxide |
| DPPH | (2,2-Diphenyl-1-picrylhydrazyl) |
| FeO | Iron oxide |
| GO | Graphene oxide |
| GO–CH | Chitosan functionalized graphene oxide |
| GO–CH–Ma | Mannose decorated chitosan functionalized graphene oxide |
| HAV | Hepatitis A virus |
| HSV | Herpes simplex virus |
| LSPR | Localized surface plasmon resonance |
| MIC | Minimum inhibitory concentration |
| MRI | Magnetic resonance imaging |
| NaOH | Sodium hydroxide |
| NP | Nanoparticle |
| Pd | Palladium |
| Pt | Platinum |
| ROS | Reactive oxygen species |
| SDS | Sodium dodecyl sulphate |
| Se | Selenium |
| SiO2 | Silicon dioxide |
| TiO2 | Titanium dioxide |
| ZnO | Zinc oxide |
Data availability
No primary research results, software or code have been included and no new data were generated or analysed as part of this review.Author contributions
Nishmitha Ramaraj: writing – original draft, methodology, conceptualization, data curation. Gobika Thiripuranathar: writing – review & editing, validation, supervision, conceptualization. Sagarika Ekanayake: writing – review & editing, validation, supervision, conceptualization. Keerthi Attanayake: writing – review & editing, validation, supervision, conceptualization. Upul Marapana: supervision, investigation.Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this article.Acknowledgements
This work was supported by the University of Sri Jayewardenepura, Sri Lanka, and the College of Chemical Sciences, Institute of Chemistry Ceylon, Sri Lanka.References
- I. Khan, K. Saeed and I. Khan, Arabian J. Chem., 2019, 12(7), 908–931 Search PubMed.
- P. G. Jamkhande, N. W. Ghule and A. H. Bamer, J. Drug Delivery Sci. Technol., 2019, 53, 101174 Search PubMed.
- N. S. Alsaiari, F. M. Alzahrani, A. Amari, H. Osman, H. N. Harharah, N. Elboughdiri and M. A. Tahoon, Molecules, 2023, 28(1), 1–17 Search PubMed.
- S. Rehman, B. R. Jermy, S. Akhtar, J. F. Borgio, S. Abdul Azeez, V. Ravinayagam, R. Al Jindan, Z. H. Alsalem, A. Buhameid and A. Gani, Artif. Cells, Nanomed., Biotechnol., 2019, 47(1), 2072–2082 Search PubMed.
- S. Adarshan, V. S. S. Sree, P. Muthuramalingam, K. S. Nambiar, M. Sevanan, L. Satish, B. Venkidasamy, P. G. Jeelani and H. Shin, Plants, 2024, 13, 113 Search PubMed.
- A. Leandro, L. Pereira and A. M. M. Gonçalves, Mar. Drugs, 2020, 18, 17 Search PubMed.
- K. Ahmad, S. Khan, M. Afridi, A. Hassan, M. M. Shah, H. Rasheed, R. Ahmad and H. Ifqir, Beni-Suef Univ. J. Basic Appl. Sci., 2022, 11(1), 156 Search PubMed.
- J. Muthukumar, R. Chidambaram and S. Sukumaran, J. Food Sci. Technol., 2021, 58, 2453–2466 Search PubMed.
- Y. Li, Y. Zheng, Y. Zhang, Y. Yang, P. Wang, B. Imre, A. C. Y. Wong, Y. S. Y. Hsieh and D. Wang, Mar. Drugs, 2021, 19, 620 Search PubMed.
- A. Dobrinčić, S. Balbino, Z. Zorić, S. Pedisić, D. Bursać Kovačević, I. Elez Garofulić and V. Dragović-Uzelac, Mar. Drugs, 2020, 18, 168 Search PubMed.
- B. Uzair, A. Liaqat, H. Iqbal, B. Menaa, A. Razzaq, G. Thiripuranathar, N. F. Rana and F. Menaa, Bioengineering, 2020, 7(4), 1–22 Search PubMed.
- L. Cassani, N. E. Marcovich and A. Gomez-Zavaglia, Crit. Rev. Food Sci. Nutr., 2023, 63(11), 1527–1550 Search PubMed.
- J. B. Moreira, T. D. Santos, C. G. Cruz, J. T. da Silveira, L. F. de Carvalho, M. G. de Morais and J. A. V. Costa, Polysaccharides, 2023, 4(4), 371–389 Search PubMed.
- B. Pradhan, P. P. Bhuyan and J.-S. Ki, Mar. Drugs, 2023, 21(5), 300 Search PubMed.
- M. Ciancia, M. C. Matulewicz and R. Tuvikene, Front. Plant Sci., 2020, 11, 559986 Search PubMed.
- F. Menaa, U. Wijesinghe, G. Thiripuranathar, N. A. Althobaiti, A. E. Albalawi, B. A. Khan and B. Menaa, Mar. Drugs, 2021, 19(9), 1–36 Search PubMed.
- P. Bhuyar, M. H. A. Rahim, S. Sundararaju, R. Ramaraj, G. P. Maniam and N. Govindan, J. Basic Appl. Sci., 2020, 9(1), 1–15 Search PubMed.
- H. Hameed, A. Waheed, M. S. Sharif, M. Saleem, A. Afreen, M. Tariq, A. Kamal, W. A. Al-Onazi, D. A. Al Farraj, S. Ahmad and R. M. Mahmoud, Micromachines, 2023, 14(5), 928 Search PubMed.
- A. S. Mahmood, Q. Saquib, V. De Matteis, H. Awad Alwathnani, S. Ali Alharbi and A. Ali Al-Khedhairy, Bioinorg. Chem. Appl., 2021, 1–26, 5985377 Search PubMed.
- N. González-Ballesteros, M. C. Rodríguez-Argüelles, S. Prado-López, M. Lastra, M. Grimaldi, A. Cavazza and F. Bigi, Mater. Sci. Eng., C, 2019, 97, 498–509 Search PubMed.
- S. Ying, Z. Guan, P. C. Ofoegbu, P. Clubb, C. Rico, F. He and J. Hong, Environ. Technol. Innovation, 2022, 26, 102336 Search PubMed.
- S. Trivedi, M. A. Alshehri, A. T. Aziz, C. Panneerselvam, H. A. Al-Aoh, F. Maggi, S. Sut and S. Dall'Acqua, S. Afr. J. Bot., 2021, 139, 432–441 Search PubMed.
- D. Franco, G. Calabrese, S. P. P. Guglielmino and S. Conoci, Microorganisms, 2022, 10, 1778 Search PubMed.
- A. Salleh, R. Naomi, N. D. Utami, A. W. Mohammad, E. Mahmoudi, N. Mustafa and M. B. Fauzi, Nanomaterials, 2020, 10(8), 1–20 Search PubMed.
- S. H. Haji, F. A. Ali and S. T. H. Aka, Sci. Rep., 2022, 12, 15254 Search PubMed.
- S. Naz, A. Gul and M. Zia, et al., Appl. Microbiol. Biotechnol., 2023, 107, 1039–1061 Search PubMed.
- R. Thiurunavukkarau, S. Shanmugam and K. Subramanian, et al., Sci. Rep., 2022, 12, 14757 Search PubMed.
- E. F. Aboelfetoh, R. A. El-Shenody and M. M. Ghobara, Environ. Monit. Assess., 2017, 189(7), 1–15 Search PubMed.
- C. Liao, Y. Li and S. C. Tjong, Int. J. Mol. Sci., 2019, 20, 449 Search PubMed.
- M. M. Rohde, C. M. Snyder and J. Sloop, et al., Part. Fibre Toxicol., 2021, 18, 37 Search PubMed.
- N. E. Eleraky, A. Allam, S. B. Hassan and M. M. Omar, Pharmaceutics, 2020, 12(2), 142 Search PubMed.
- P. Senthilkumar, L. Surendran and B. Sudhagar, et al., SN Appl. Sci., 2019, 1, 284 Search PubMed.
- F. Arockiya Aarthi Rajathi, C. Parthiban, V. Ganesh Kumar and P. Anantharaman, Spectrochim. Acta, Part A, 2012, 99, 166–173 Search PubMed.
- Y. Zhang, T. P. Shareena Dasari, H. Deng and H. Yu, J. Environ. Sci. Health, Part C: Environ. Carcinog. Ecotoxicol. Rev., 2015, 33(3), 286–327 Search PubMed.
- Y. N. Slavin, J. Asnis, U. O. Häfeli and H. Bach, J. Nanobiotechnol., 2017, 15(1), 1–20 Search PubMed.
- K. P. Anjali, B. M. Sangeetha, R. Raghunathan, G. Devi and S. Dutta, ChemistrySelect, 2021, 6(4), 647–656 Search PubMed.
- A. Fouda, A. M. Eid, A. Abdelkareem, H. A. Said, E. F. El-Belely, D. H. M. Alkhalifah, K. S. Alshallash and S. E. D. Hassan, Catalysts, 2022, 12(7), 756 Search PubMed.
- A. K. Hajri, M. A. Albalawi, I. Alsharif and B. Jamoussi, Bioinorg. Chem. Appl., 2022, 1–11, 3977935 Search PubMed.
- R. Amjad, B. Mubeen, S. S. Ali, S. S. Imam, S. Alshehri, M. M. Ghoneim, S. I. Alzarea, R. Rasool, I. Ullah, M. S. Nadeem and I. Kazmi, Polymers, 2021, 13(24), 4364 Search PubMed.
- T. Balaji, C. M. Manushankar, K. A. Al-Ghanim, C. Kamaraj, D. Thirumurugan, S. Thanigaivel, M. Nicoletti, N. Sachivkina and M. Govindarajan, Biomedicines, 2023, 11(8), 2285 Search PubMed.
- Y. Abboud, T. Saffaj, A. Chagraoui, A. El Bouari, K. Brouzi, O. Tanane and B. Ihssane, Appl. Nanosci., 2014, 4(5), 571–576 Search PubMed.
- M. C. Fisher, A. Alastruey-Izquierdo, J. Berman, T. Bicanic, E. M. Bignell, P. Bowyer and P. E. Verweij, Nat. Rev. Microbiol., 2022, 20(9), 557–571 Search PubMed.
- S. Mickymaray and W. Alturaiki, Molecules, 2018, 23(11), 1–14 Search PubMed.
- A. Shojaee, A. Jahandideh, A. Nasrollahi Omran, N. Sohrabi Haghdoost and M. Khosravi, Curr. Med. Mycol., 2023, 9(1), 14–20 Search PubMed.
- R. A. Hamouda, F. Q. Almaghrabi, O. M. Alharbi, A. D. M. Al-Harbi, R. M. Alsulami and A. M. Alhumairi, Mar. Drugs, 2024, 22, 225 Search PubMed.
- A. Aggarwal, M. P. S. Chawla and J. Indian Acad, Clin. Med., 2021, 22(3–4), 117–132 Search PubMed.
- P. Kumar, S. S. Senthamil Selvi and M. Govindaraju, Appl. Nanosci., 2013, 3(6), 495–500 Search PubMed.
- A. Pugazhendhi, R. Prabhu, K. Muruganantham, R. Shanmuganathan and S. Natarajan, J. Photochem. Photobiol. B: Biol., 2019, 190, 86–97 Search PubMed.
- M. A. Gatou, E. Skylla, P. Dourou, N. Pippa, M. Gazouli, N. Lagopati and E. A. Pavlatou, Crystals, 2024, 14(3), 215 Search PubMed.
- S. N. Eladl, A. M. Elnabawy and E. G. Eltanahy, Bot. Stud., 2024, 65, 28 Search PubMed.
- M. El-Sheekh, S. S. AlKafaas, H. Rady, B. E. Abdelmoaty, H. M. Bedair, A. A. Ahmed, M. T. El-Saadony, S. F. AbuQamar and K. A. El-Tarabily, Int. J. Nanomed., 2023, 18, 6601–6638 Search PubMed.
- S. Jeyarani, N. M. Vinita, P. Puja, S. Senthamilselvi, U. Devan, A. J. Velangani, M. Biruntha, A. Pugazhendhi and P. Kumar, J. Photochem. Photobiol., B, 2020, 202, 111715 Search PubMed.
- N. M. Aboeita, S. A. Fahmy, M. M. H. El-Sayed, H. M. E.-S. Azzazy and T. Shoeib, Pharmaceutics, 2022, 14, 418 Search PubMed.
- B. Babu, S. Palanisamy, M. Vinosha, R. Anjali, P. Kumar, B. Pandi, M. Tabarsa, S. G. You and N. M. Prabhu, Bioprocess Biosyst. Eng., 2020, 43(12), 2231–2242 Search PubMed.
- R. Kimura, T. Rokkaku, S. Takeda, M. Senba and N. Mori, Mar. Drugs, 2013, 11(11), 4267–4278 Search PubMed.
- J. Sarkar, S. Das, S. Aich, P. Bhattacharyya and K. Acharya, J. Trace Elem. Med. Biol., 2022, 72, 126977 Search PubMed.
- R. G. Bai and R. Tuvikene, Viruses, 2021, 13(9), 1817 Search PubMed.
- J. A. Panggabean, S. P. Adiguna, S. I. Rahmawati, P. Ahmadi, E. N. Zainuddin, A. Bayu and M. Y. Putra, Molecules, 2022, 27, 1178 Search PubMed.
- C. Wijesinghe, J. Gunatilake and P. H. D. Kusumawathie, et al., Parasites Vectors, 2021, 14, 614 Search PubMed.
- P. Orłowski, A. Kowalczyk, E. Tomaszewska, K. Ranoszek-Soliwoda, A. Węgrzyn, J. Grzesiak, G. Celichowski, J. Grobelny, K. Eriksson and M. Krzyzowska, Viruses, 2018, 10(10), 524 Search PubMed.
- R. Kothai, B. Arul and V. Anbazhagan, Appl. Biochem. Biotechnol., 2023, 195(6), 3747–3763 Search PubMed.
- H. E. Touliabah, M. M. El-Sheekh and M. E. M. Makhlof, Front. Mar. Sci., 2022, 9, 1092343 Search PubMed.
- V. Govindarajan, J. P. de Rivero Vaccari and R. W. Keane, J. Neuroinflammation, 2020, 17, 260 Search PubMed.
- D. S. Ghallab, R. S. Ibrahim, M. M. Mohyeldin and E. Shawky, Mar. Pollut. Bull., 2024, 199, 116023 Search PubMed.
- A. S. Almurshedi, T. A. El-Masry, H. Selim, M. M. El-Sheekh, M. E. M. Makhlof, B. N. Aldosari, I. M. Alfagih, B. T. AlQuadeib, S. S. Almarshidy and M. M. El-Bouseary, Microb. Cell Fact., 2023, 22(1), 1–19 Search PubMed.
- M. N. A. Azeem, O. M. Ahmed, M. Shaban and K. N. M. Elsayed, Environ. Sci. Pollut. Res., 2022, 29(39), 59930–59947 Search PubMed.
- H. Rosi, R. Ethrajavalli and M. I. Janci, International Conference on Systems, Computation, Automation Networking (ICSCAN), 2020, pp. 22–24 Search PubMed.
- C. Jimenez-Lopez, A. G. Pereira, C. Lourenço-Lopes, P. García-Oliveira, L. Cassani, M. Fraga-Corral and J. Simal-Gandara, Food Chem., 2021, 341, 128262 Search PubMed.
- Y. Athukorala, K. N. Kim and Y. J. Jeon, Food Chem. Toxicol., 2006, 44(7), 1065–1074 Search PubMed.
- J. Venkatesan, S. K. Kim and M. S. Shim, Nanomaterials, 2016, 6(12), 235 Search PubMed.
- G. Sudha and A. Balasundaram, J. Nanosci. Technol., 2018, 4(4), 424–426 Search PubMed.
- A. D. Solanki and I. Patel, Egpt. J. Agric. Res., 2022, 100(3), 394–401 Search PubMed.
- R. A. Hamouda and E. S. Aljohani, Mar. Drugs, 2024, 22(4), 154 Search PubMed.
- M. Fernandes, N. González-Ballesteros, A. da Costa, R. Machado, A. C. Gomes and M. C. Rodríguez-Argüelles, J. Biol. Inorg. Chem., 2023, 28(4), 439–450 Search PubMed.
- S. Rajeshkumar, C. Malarkodi, M. Vanaja, G. Gnanajobitha, K. Paulkumar, C. Kannan and G. Annadurai, Pharma Chem., 2013, 5(2), 224–229 Search PubMed.
- R. Thiruchelvi, P. Jayashree and K. Mirunaalini, Mater. Today: Proc., 2020, 37(Part 2), 1693–1698 Search PubMed.
- S. Rajeshkumar, C. Kannan and G. Annadurai, Drug Invent. Today, 2012, 4(10), 511–513 Search PubMed.
- C. M. Ramakritinan, E. Kaarunya, S. Shankar and A. K. Kumaraguru, Adv. Mater. Res., 2013, 698, 211–230 Search PubMed.
- N. Supraja, T. N. V. K. V. Prasad, M. Soundariya and R. Babujanarthanam, AIMS Bioeng., 2016, 3(4), 425–440 Search PubMed.
- R. Algotiml, A. Gab-Alla, R. Seoudi, H. H. Abulreesh, M. Z. El-Readi and K. Elbanna, Sci. Rep., 2022, 12(1), 1–18 Search PubMed.
- S. Rajeshkumar, R. P. Parameswari, J. Jayapriya, M. Tharani, H. Ali, N. H. Aljarba, S. Alkahtani and S. Alarifi, Biomed. Res. Int., 2022, 5746761 Search PubMed.
- M. Singh, K. Saurav, A. Majouga, M. Kumari, M. Kumar, S. Manikandan and A. K. Kumaraguru, Biotechnol. Appl. Biochem., 2015, 62(3), 424–432 Search PubMed.
- D. Acharya, Biol. Trace Elem. Res., 2021, 199(5), 1812–1822 Search PubMed.
- R. Algotiml, A. Gaballa, R. Seoudi, H. H. Abulreesh, I. Ahmad and K. Elbanna, J. Pure Appl. Microbiol., 2022, 16(1), 207–225 Search PubMed.
- K. S. Khalifa, R. A. Hamouda, D. Hanafy and A. Hamza, Dig. J. Nanomater. Biostruct., 2016, 11(1), 213–221 Search PubMed.
- J. L. Lopez-Miranda, G. A. Molina, M. A. González-Reyna, B. L. España-Sánchez, R. Esparza, R. Silva and M. Estévez, Int. J. Mol. Sci., 2023, 24(2), 1474 Search PubMed.
- J. J. Xu, W. C. Zhang, Y. W. Guo, X. Y. Chen and Y. N. Zhang, Drug Delivery, 2022, 29(1), 664–678 Search PubMed.
- E. K. Sher, M. Alebić, M. Marković Boras, E. Boškailo, E. Karahmet Farhat, A. Karahmet, B. Pavlovic, F. Sher and L. Lekic, Int. J. Pharm., 2024, 124345 Search PubMed.
- S. A. Mir, L. Hamid, G. N. Bader, A. Shoaib, M. Rahamathulla, M. Y. Alshahrani, P. Alam and F. Shakeel, Molecules, 2022, 27, 6608 Search PubMed.
- W. Huang, G. Xiao, Y. Zhang and W. Min, Biomed. Pharmacother., 2021, 139, 111541 Search PubMed.
- B. S. Negreanu-Pirjol, T. Negreanu-Pirjol, D. R. Popoviciu, R. E. Anton and A. M. Prelipcean, Pharmaceutics, 2022, 14(9), 1781 Search PubMed.
- P. Manivasagan, S. Bharathiraja, N. Q. Bui, B. Jang, Y. O. Oh, I. G. Lim and J. Oh, Int. J. Biol. Macromol., 2016, 91, 578–588 Search PubMed.
- R. Jayakumar, M. Prabaharan, S. V. Nair and H. Tamura, Biotechnol. Adv., 2010, 28(1), 142–150 Search PubMed.
- A. I. Barbosa, A. J. Coutinho, S. A. Costa Lima and S. Reis, Mar. Drugs, 2019, 17(12), 654 Search PubMed.
- S. Kesavan, K. S. Meena, S. A. Sharmili, M. Govindarajan, N. S. Alharbi, S. Kadaikunnan, J. M. Khaled, A. S. Alobaidi, K. F. Alanzi and B. Vaseeharan, J. Drug Deliv. Sci. Technol., 2021, 65, 102760 Search PubMed.
- M. Nurunnabi, K. Parvez, M. Nafiujjaman, V. Revuri, H. A. Khan, X. Feng and Y. K. Lee, RSC Adv., 2015, 5(52), 42141–42161 Search PubMed.
- S. Ye, C. Xie, O. T. Agar, C. J. Barrow, F. R. Dunshea and H. A. R. Suleria, Food Rev. Int., 2023, 1–29, 2682–2710 Search PubMed.
- P. Severino, C. F. da Silva, L. N. Andrade, D. de Lima Oliveira, J. Campos and E. B. Souto, Curr. Pharm. Des., 2019, 25(11), 1312–1334 Search PubMed.
- M. Singh, M. Kumar, S. Manikandan, N. Chandrasekaran, A. Mukherjee and A. K. Kumaraguru, J. Nanomed. Nanotechnol., 2014, 5, 009 Search PubMed.
- S. J. Wu, T. M. Don, C. W. Lin and F. L. Mi, Mar. Drugs, 2014, 12(11), 5677–5697 Search PubMed.
- Z. Štukovnik, R. Fuchs-Godec and U. Bren, Biosensors, 2023, 13(10), 899 Search PubMed.
- H. Zhao, Y. Wang, L. Bao and C. Chen, Acc. Mater. Res., 2022, 3(8), 812–829 Search PubMed.
- S. Đorđević, M. M. Gonzalez, I. Conejos-Sánchez, B. Carreira, S. Pozzi, R. C. Acúrcio, R. Satchi-Fainaro, H. F. Florindo and M. J. Vicent, Drug Delivery Transl. Res., 2022, 12, 1–26 Search PubMed.
- J. Li, X. Gao, Y. Wang, T. Xia, Y. Zhao and H. Meng, Matter, 2022, 5(4), 1162–1191 Search PubMed.
- Y. H. Hsueh, K. S. Lin, W. J. Ke, C. T. Hsieh, C. L. Chiang, D. Y. Tzou and S. T. Liu, PLoS One, 2015, 10(12), e0144306 Search PubMed.
- C. Liao, Y. Li and S. C. Tjong, Int. J. Mol. Sci., 2019, 20(2), 449 Search PubMed.
- Z. M. Xiu, Q. B. Zhang, H. L. Puppala, V. L. Colvin and P. J. Alvarez, Nano Lett., 2012, 12(8), 4271–4275 Search PubMed.
- M. Akter, M. T. Sikder, M. M. Rahman, A. K. M. A. Ullah, K. F. B. Hossain, S. Banik, T. Hosokawa, T. Saito and M. Kurasaki, J. Adv. Res., 2017, 9, 1–16 Search PubMed.
- C. Egbuna, V. K. Parmar, J. Jeevanandam, S. M. Ezzat, K. C. Patrick-Iwuanyanwu, C. O. Adetunji and C. G. Ibeabuchi, J. Toxicol., 2021, 2021, 9954443 Search PubMed.
| This journal is © The Royal Society of Chemistry 2025 |